Copyright
©The Author(s) 2016.
World J Gastroenterol. Oct 28, 2016; 22(40): 8967-8977
Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8967
Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8967
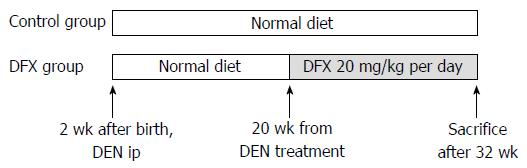
Figure 1 Protocol for the murine model of hepatocarcinogenesis.
The model was induced by injection of 10 μg/g of DEN at 14 d of age. In the deferasirox (DFX) group, 20 mg/kg of DFX was administered orally for 3 mo and fed with normal diet. In the control group, the same amount of normal diet was administered. After 3 mo (at week 32), the mice were sacrificed and underwent autopsy examination.
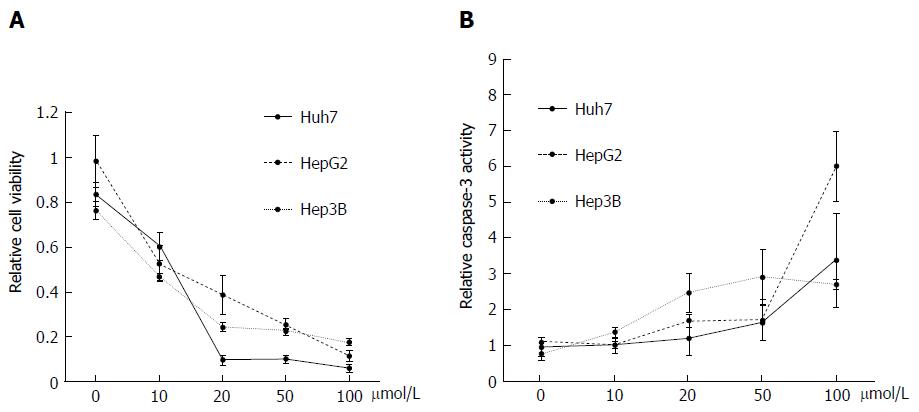
Figure 2 Antitumor effects of deferasirox in hepatoma cell lines.
A: Deferasirox (DFX) exhibited antiproliferative effects against each cell line in a dose-dependent manner as revealed by MTT assay. Bars represent SD; B: Colorimetric assay of caspase-3 activity showing activation of caspase-3 by DFX in a dose-dependent manner. Bars represent SD.
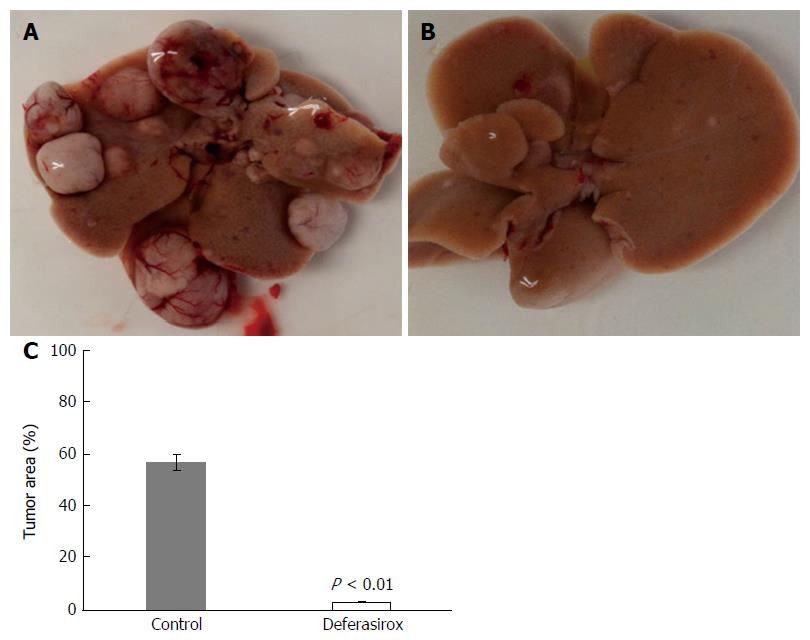
Figure 3 Inhibition of liver tumor formation by deferasirox.
Macroscopic images of liver tumors in the (A) control and (B) deferasirox (DFX) groups. Evaluation of (C) tumor area percentage of the total specimen area in control and DFX-treated mice. Bars represent SD.
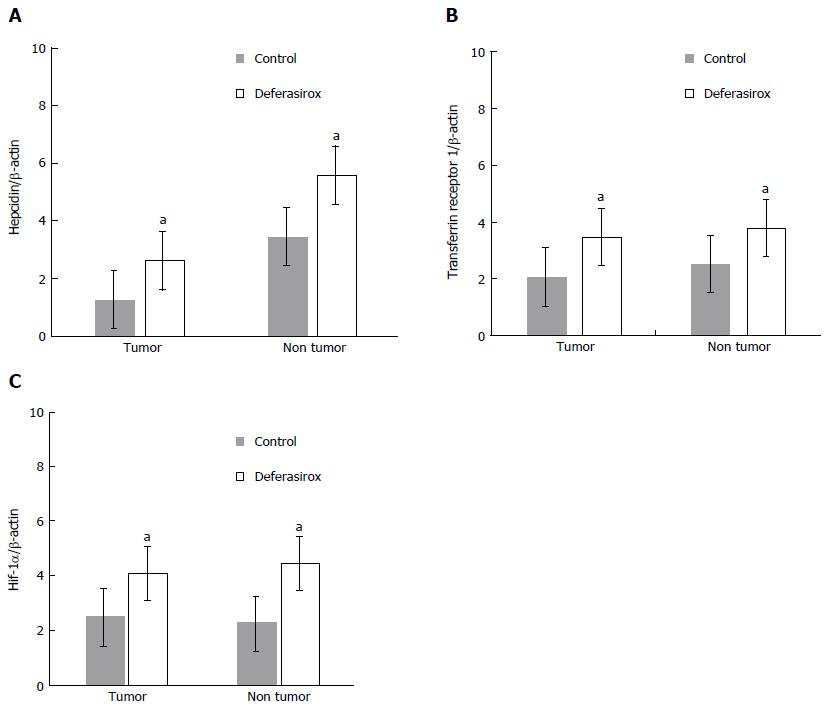
Figure 4 Regulation of iron-related gene expressions by deferasirox in a murine model.
Real-time RT-PCR data of the expressions of (A) hepcidin, (B) transferrin receptor 1, and (C) HIF-1α in tumor and non-tumor tissues. Bars indicate SD. aP < 0.05.
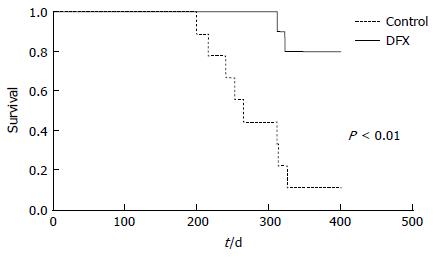
Figure 5 Cumulative survival rates of control and deferasirox-treated mice in a murine hepatocellular carcinoma model.
Deferasirox (DFX)-treated mice showed significantly higher survival rate than control mice (P < 0.01).
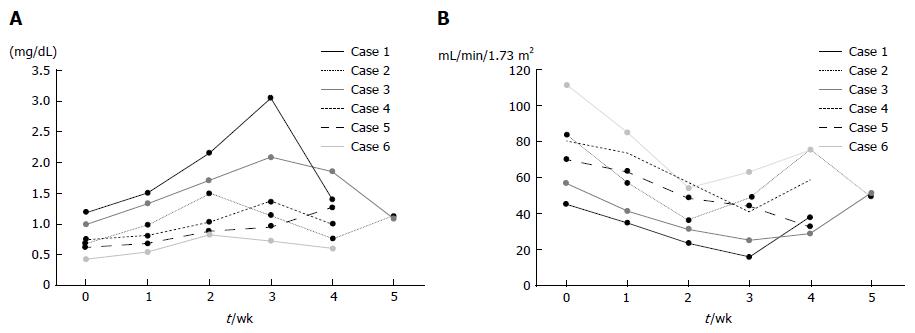
Figure 6 Changes in serum creatinine levels and eGFR of patients administered with deferasirox.
A: All patients showed gradual increase in creatinine levels after initiation of DFX treatment, until reduction in DFX dose or discontinuation of treatment. Creatinine levels in all patients were improved by dose reduction. B: All patients showed gradual decrease in eGFR after initiation of DFX treatment, until reduction in DFX dose or discontinuation of treatment.
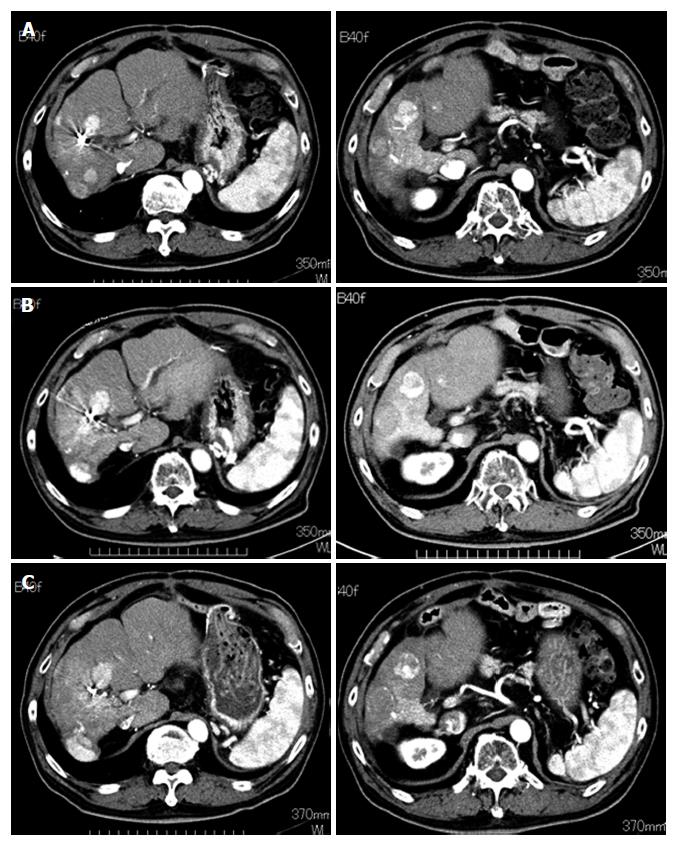
Figure 7 Progress of hepatocellular carcinoma in one patient (case 3) who maintained stable disease for eight months.
The tumor size did not increase during the DFX administration period. A: Before deferasirox (DFX) administration; B: After one course of DFX therapy; C: After four courses of DFX therapy.
- Citation: Saeki I, Yamamoto N, Yamasaki T, Takami T, Maeda M, Fujisawa K, Iwamoto T, Matsumoto T, Hidaka I, Ishikawa T, Uchida K, Tani K, Sakaida I. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J Gastroenterol 2016; 22(40): 8967-8977
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8967.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8967