Copyright
©The Author(s) 2016.
World J Gastroenterol. Jan 28, 2016; 22(4): 1664-1673
Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1664
Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1664
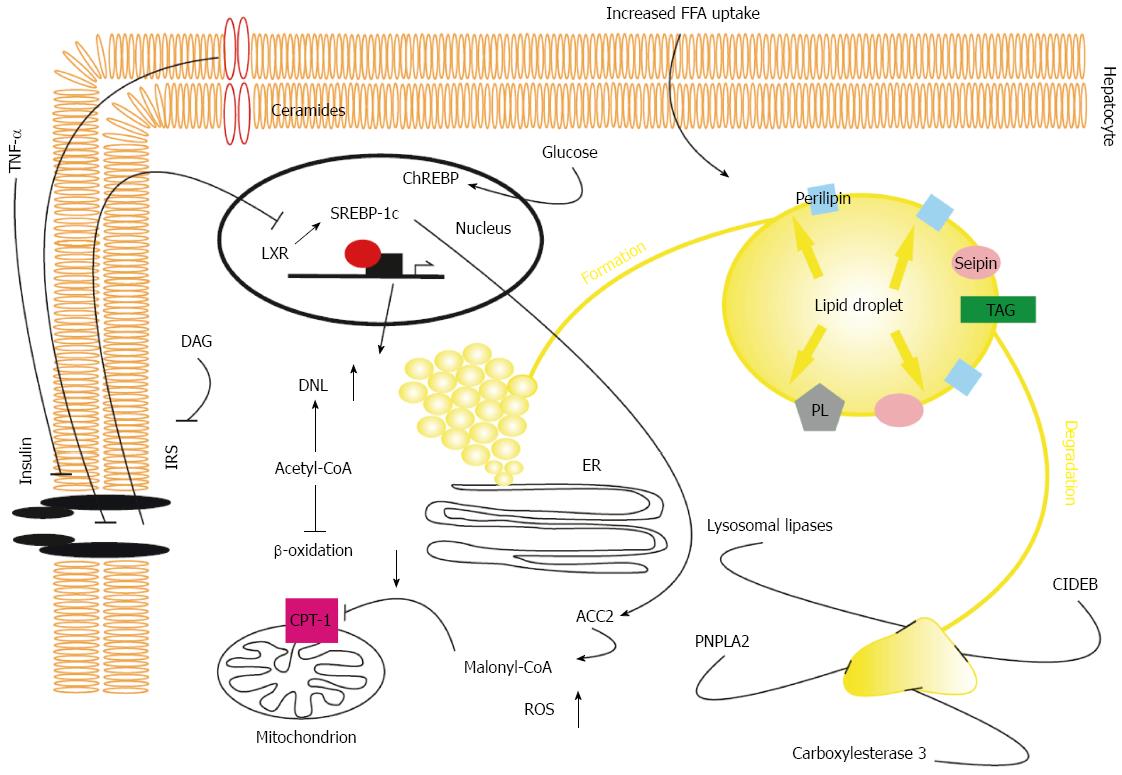
Figure 1 Pathophysiological aspects of hepatic triglyceride accumulation in non-alcoholic fatty liver disease.
Both increased uptake of fatty acids due to elevated whole body lipolysis in states of insulin resistance and enhanced fatty acid synthesis are key features of non-alcoholic fatty liver disease. Increased de novo lipogenesis results from enhanced activation of LXR, SREBP-1c, and ChREBP in insulin resistance. SREBP-induced activation of ACC2 leads to accumulation of malonyl-CoA, which in turn inhibits CPT-1 activity, resulting in reduced β-oxidation. In the liver, triglycerides are stored in LDs that are formed within the lipid bilayer of the ER. Stabilization and growth of LDs are dependent on transmembrane proteins, seipin, and triglyceride and phospholipid synthetic enzymes that are located on the LD surface. LDs are catabolized via ATGL-dependent hydrolysis and β-oxidation of fatty acids, lysosomal lipases, and carboxylesterase 3- and CIDEB- mediated repacking of cytosolic LDs in the ER, leading to synthesis of VLDL particle. Intermediates of long chain fatty acids (DAG) inhibit insulin signalling further, exacerbating hepatic insulin resistance by exerting proinflammatory effects and reducing activation of the insulin receptor. LXRα: Ligand-activated transcription factor α; SREBP1c: Sterol regulating element binding protein 1c; CPT-1: Carnitine palmitoyl transferase 1; LD: Lipid droplet; ATGL: Adipose triglyceride lipase; CIDEB: Cell death inducing DFFA like effector B; DAG: Diacylglycerol; IRS: Insulin receptor substrate; ER: Endoplasmic reticulum; ChREBP: Carbohydrate response element binding protein; LXR: Ligand-activated transcription factor; DNL: De novo lipogenesis; ACC2: Acetyl-CoA carboxylase 2; PNPLA2: Patatin-like phospholipase domain-containing protein 2; ROS: Reactive oxygen species.
- Citation: Ress C, Kaser S. Mechanisms of intrahepatic triglyceride accumulation. World J Gastroenterol 2016; 22(4): 1664-1673
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1664.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1664