Copyright
©The Author(s) 2016.
World J Gastroenterol. Sep 21, 2016; 22(35): 8050-8059
Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.8050
Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.8050
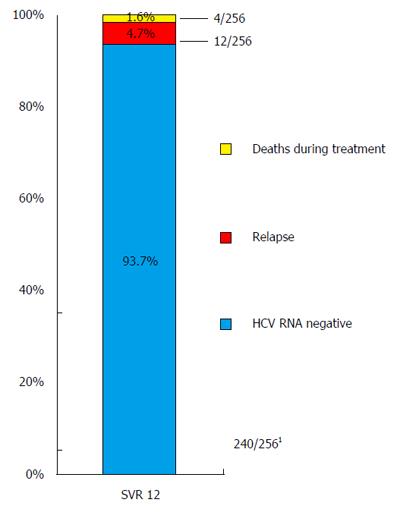
Figure 1 Overall treatment outcome.
1Two hundred and sixty consecutive patients were treated with second generation direct acting antiviral (DAA) combinations; 14/260 patients lost to follow-up were not included in our analysis [modified intention-to-treat (mITT) analysis]. Additionally, 2 patients discontinued treatment prematurely, but achieved sustained virological response (SVR); accordingly these 2 patients also were counted as SVR12 patients.
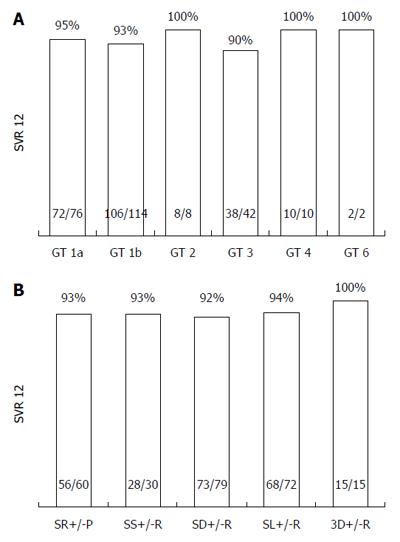
Figure 2 SVR12 rates with respect to GT (A), and diverse direct acting antiviracombinations (B, both modified intention-to-treat).
This mITT analysis excluded patients, who were lost to follow-up (n = 4). Additionally, three GT1x patients and one GT 5 patient achieved SVR12. For further subgroup analyses according to baseline parameters see Table 2. 3D: Dasabuvir, Ombitasvir, and Paritaprevir/r; GT: Genotype; GT1x: Genotype 1, no subtype differentiable; P: Pegylated Interferon; R: Ribavirin; SR: Sofosbuvir, Ribavirin; SS: Sofosbuvir, Simeprevir; SL: Sofosbuvir, Ledipasvir; SD: Sofosbuvir, Daclatasvir; SVR: Sustained virological response.
- Citation: Werner CR, Schwarz JM, Egetemeyr DP, Beck R, Malek NP, Lauer UM, Berg CP. Second-generation direct-acting-antiviral hepatitis C virus treatment: Efficacy, safety, and predictors of SVR12. World J Gastroenterol 2016; 22(35): 8050-8059
- URL: https://www.wjgnet.com/1007-9327/full/v22/i35/8050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i35.8050