Copyright
©The Author(s) 2016.
World J Gastroenterol. Aug 14, 2016; 22(30): 6936-6943
Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6936
Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6936
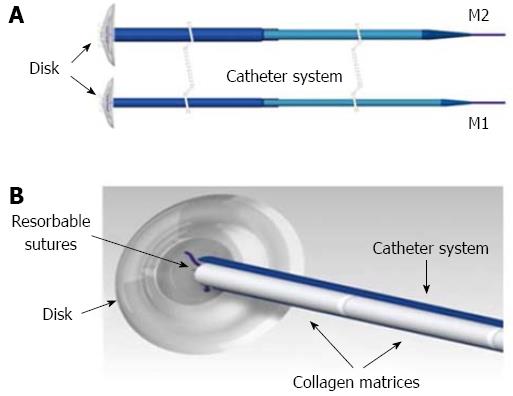
Figure 1 M1 and M2 Curaseal AF™ device.
A disk of silicone is joined to a delivery catheter system containing 6 collagen matrices that are released in the fistula tract (A); the disk is expelled when the resorbable sutures degrade, and the collagen matrices provide a scaffold during the healing process (B).
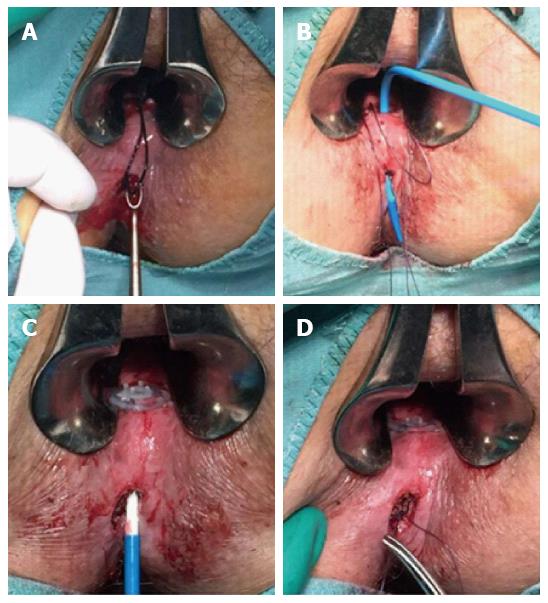
Figure 2 The procedure.
The fistula tract was debrided by curetting (A); the device was placed in the fistula tract via the internal fistula opening (B); the collagen matrices were unsheated in the fistula tract and the disk was sutured to the internal opening (C); the external matrix was sutured to the external opening, which was left open (D).
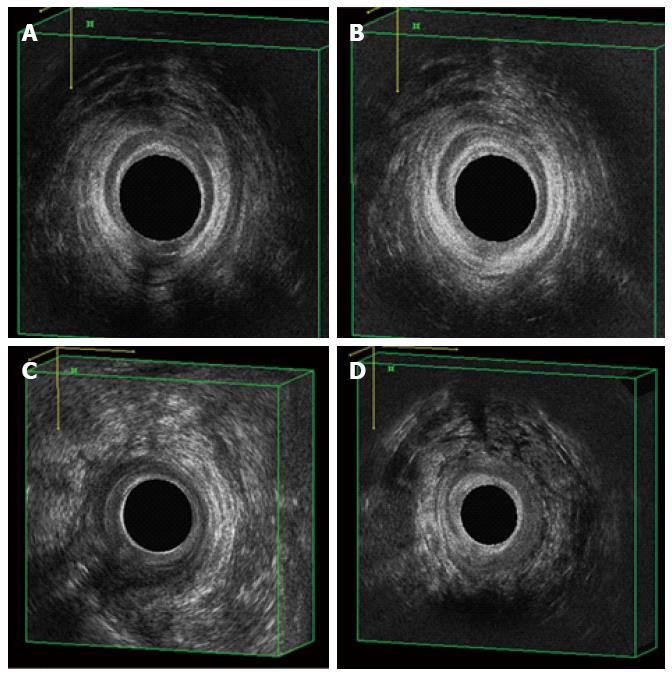
Figure 3 Pre- and post-operative evaluation by 3D-endoanal ultrasound.
A posterior transphincteric fistula (A), healed at the 6-mo FU visit (B); a right posterior-lateral extrasphincteric fistula (C), healed at the 6-mo FU visit (D). FU: Follow up.
- Citation: Ratto C, Litta F, Donisi L, Parello A. Prospective evaluation of a new device for the treatment of anal fistulas. World J Gastroenterol 2016; 22(30): 6936-6943
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6936.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6936