Copyright
©The Author(s) 2016.
World J Gastroenterol. Jul 28, 2016; 22(28): 6469-6483
Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6469
Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6469
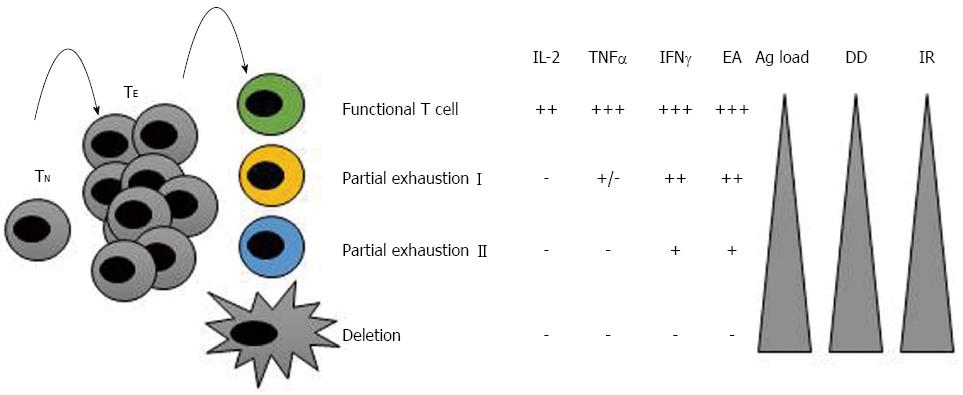
Figure 1 T cell exhaustion during diseases with persistent and high antigenemia.
At the beginning of an infection, naïve T cells (TN) are primed and differentiate into effector T cells (TE). During acute infections, TE are completely functional and control the pathogen/tumoural cell. After clearing the antigen, these cells are then deleted by apoptosis and a memory population is generated and maintained. Nevertheless, in conditions of chronic infections or tumours, these cells gradually loss their effector capacity, becoming exhausted. The greater the antigen load or duration of the infection, the more exhausted the cells become. The steps of exhaustion are summarised here. In “partial exhaustion I” IL-2 production, high expansion ability and ex vivo killing are lost. In “partial exhaustion II”, the more advanced stage of exhaustion, these cells lose their capacity to produce tumour necrosis factor (TNF)-α, produce less interferon-γ and proliferate less. In the final stage of exhaustion, these cells are deleted by apoptosis. Ag: Antigen; DD: Duration of the disease; EA: Expansion ability; IR: Inhibitory receptors’ expression.
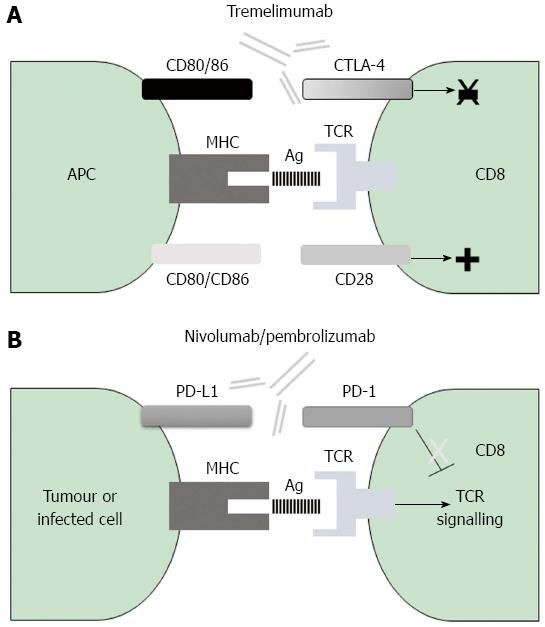
Figure 2 Mechanisms of action of negative co-stimulatory pathways involved in current immunotherapy for cytotoxic T cells.
A: The CTLA-4 immune checkpoint impairs early T cell activation; the inhibitory CTLA-4 molecule binds with higher affinity to the CD80/CD86 ligands on antigen presenting cells and prevents their binding to the positive co-stimulatory molecule CD28, with the consequence being a decrease in T cell activation. B: The PD-1 negative receptor on T cells interacts with either PD-L1 or PD-L2 on the surface of the infected or tumoural cell, thereby promoting T cell exhaustion; blockade of either CTLA-4 or PD-1 with humanized monoclonal antibodies releases the break that is exerted by these molecules and results in T cell activation. CTLA-4: Cytotoxic T-lymphocyte antigen-4; MHC: Major histocompatibility complex; PD-1: Programmed cell death protein-1; TCR: T cell receptor.
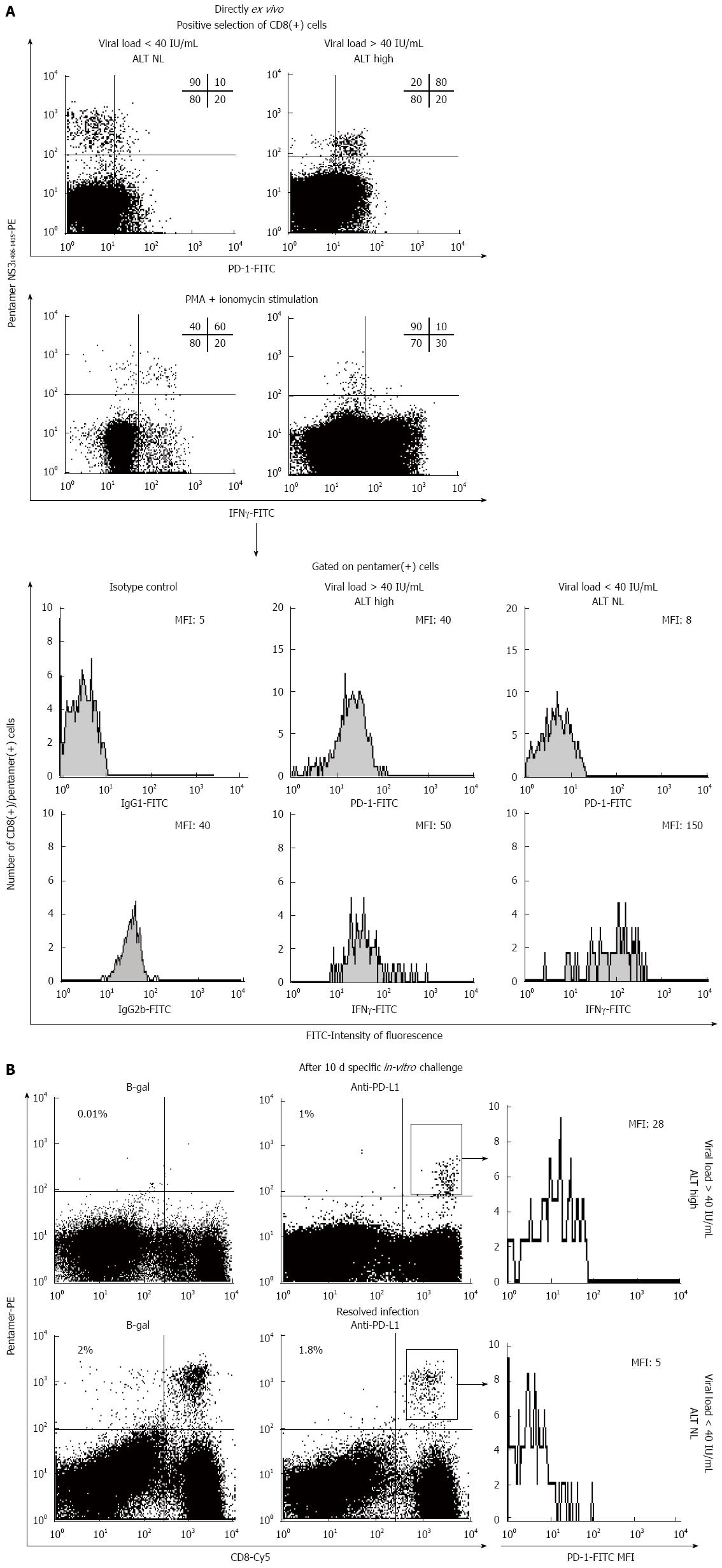
Figure 3 PD-1/PD-L1 blockade-induced restoration in vitro of hepatitis C virus-specific CD8+ T cells from a patient with chronic hepatitis C virus infection.
A: FACScan® dot-plots directly gated on ex vivo CD8+ T cells showing the PD-1 phenotype and the interferon (IFN)-γ secretion of HCV-NS31406-pentamer-binding CD8+ T cells from a patient with chronic HCV infection and a patient who was able to resolve the infection; B: FACScan® dot-plots showing the frequency of HCV-NS31406-pentamer-binding CD8+ T cells after a 10-d specific in vitro challenge in the presence and absence of anti-PD-L1 to block the interaction between PD-1 and its ligand in the patients from (A) with chronic HCV infection and who resolved the infection; PD-1 expression on gated HCV-NS31406-pentamer-binding CD8+ T cells after expansion is also shown. ALT: Alanine aminotransferase; HCV: Hepatitis C virus.
- Citation: Moreno-Cubero E, Larrubia JR. Specific CD8+ T cell response immunotherapy for hepatocellular carcinoma and viral hepatitis. World J Gastroenterol 2016; 22(28): 6469-6483
- URL: https://www.wjgnet.com/1007-9327/full/v22/i28/6469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i28.6469