Copyright
©The Author(s) 2016.
World J Gastroenterol. Jul 14, 2016; 22(26): 6036-6048
Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6036
Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6036
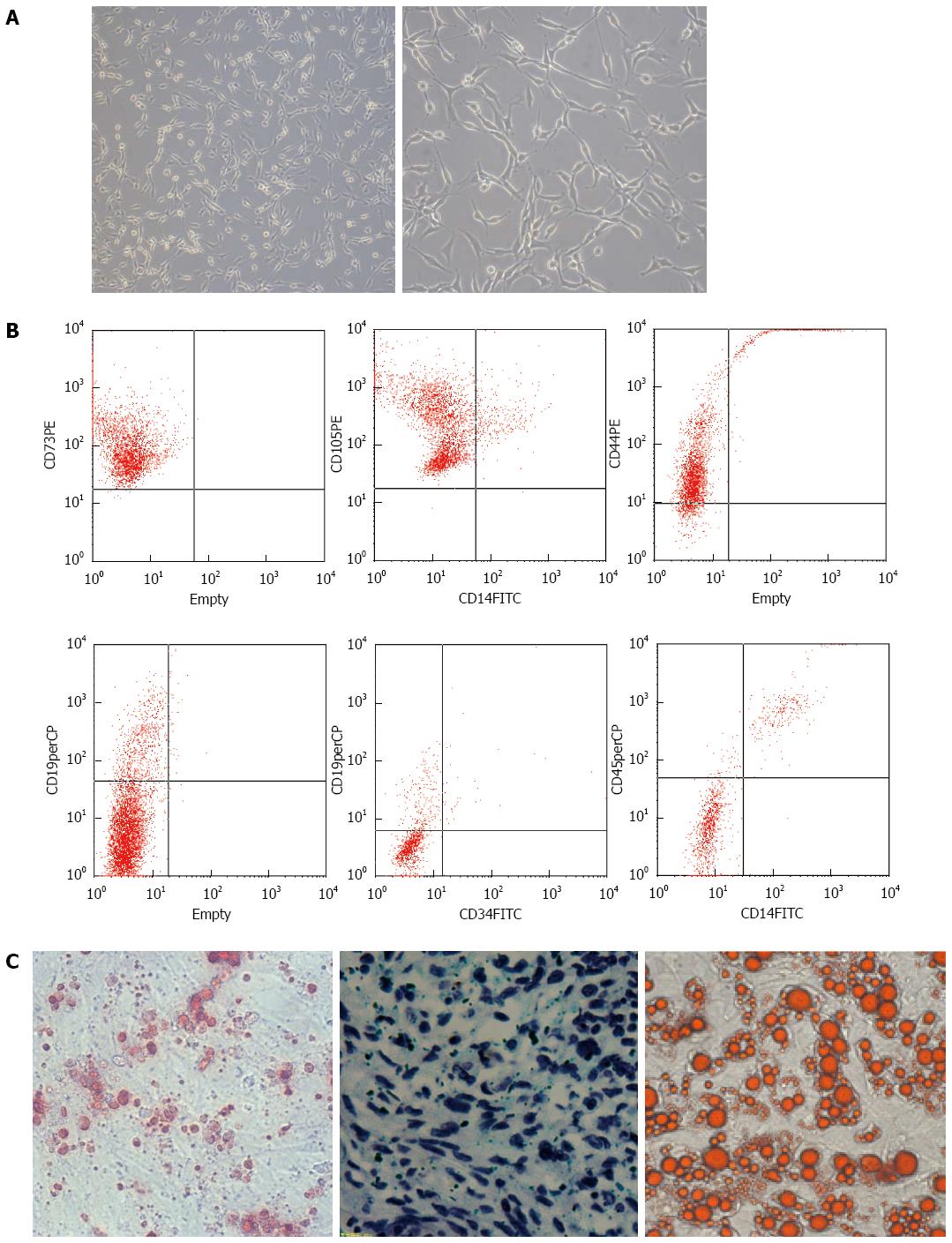
Figure 1 Mesenchymal stem cells were isolated and purified successfully.
A: Mesenchymal stem cells (MSCs) are long spindle-shaped cells (right, magnification × 40); B: Flow cytometry showing that umbilical cord derived MSCs express high levels of CD73, CD105 and CD44, but almost no CD19, CD34, CD45 and CD14; C: The purified MSCs were capable of osteogenesis (left, magnification × 40, by Alizarin Red), chondrogenesis (middle, magnification × 40, by Toluidine Blue O) and adipogenesis (right, magnification × 40, by Oil Red-O).
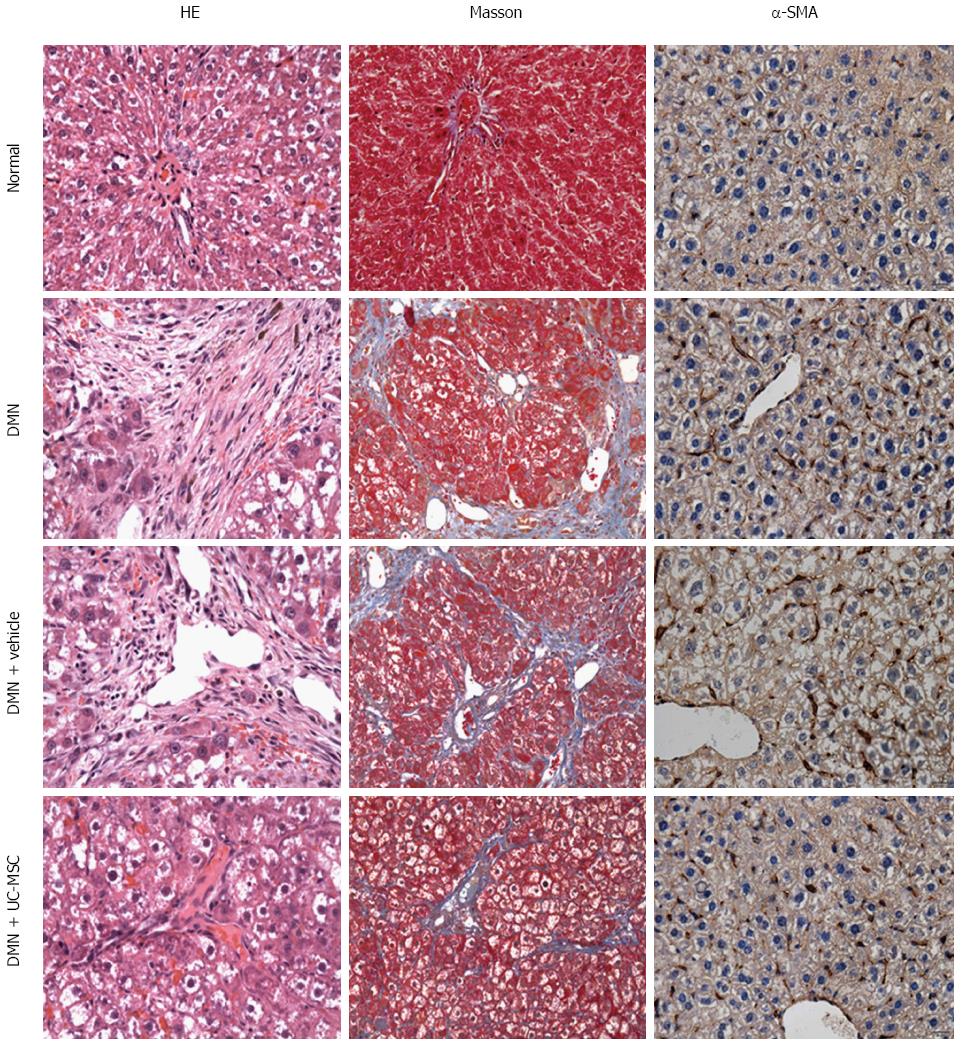
Figure 2 Umbilical cord-derived mesenchymal stem cells transplantation alleviates liver fibrosis.
Hematoxylin and eosin (HE) staining of liver sections showing massive vacuolar degeneration of hepatocytes and intense neutrophil infiltration in DMN-treated rats and control rats (first column, HE staining). Masson’s trichrome staining showed periportal fibrosis with fibrous septa extending into adjacent portal tracts and the terminal hepatic venule in DMN-treated rats and DMN + Vehicle animals (second column, Masson staining), suggesting the successful establishment of the animal model of hepatic fibrosis. Liver fibrosis and inflammation were significantly decreased by transfusion with UC-MSCs in UC-MSC-treated rats compared with the DMN + Vehicle group, which was characterized by short septa extending into lobules or portal-portal septa (first column, HE staining, and second column, Masson staining). Alpha-SMA is a marker of activated hepatic stellate cells, which plays key roles in the pathogenesis of liver fibrosis. Increased expression of α-SMA was observed in the model and DMN + Vehicle groups, and was decreased by UC-MSCs transfusion (third column, α-SMA immunohistochemical staining).
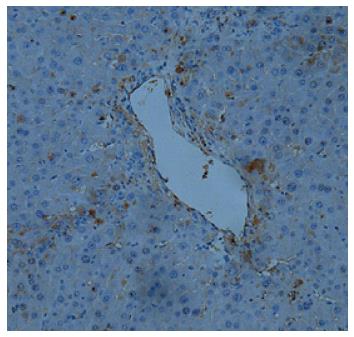
Figure 3 Umbilical cord-derived mesenchymal stem cells transfused into rats are recruited into the injured liver.
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) transfused into fibrotic liver animals were labeled with green fluorescent protein (GFP). Immunohistochemical detection with anti-GFP was performed to assess the distribution of UC-MSCs in the liver after DMN treatment. UC-MSCs were located in the injured liver together with infiltrated inflammatory cells (magnification × 40).
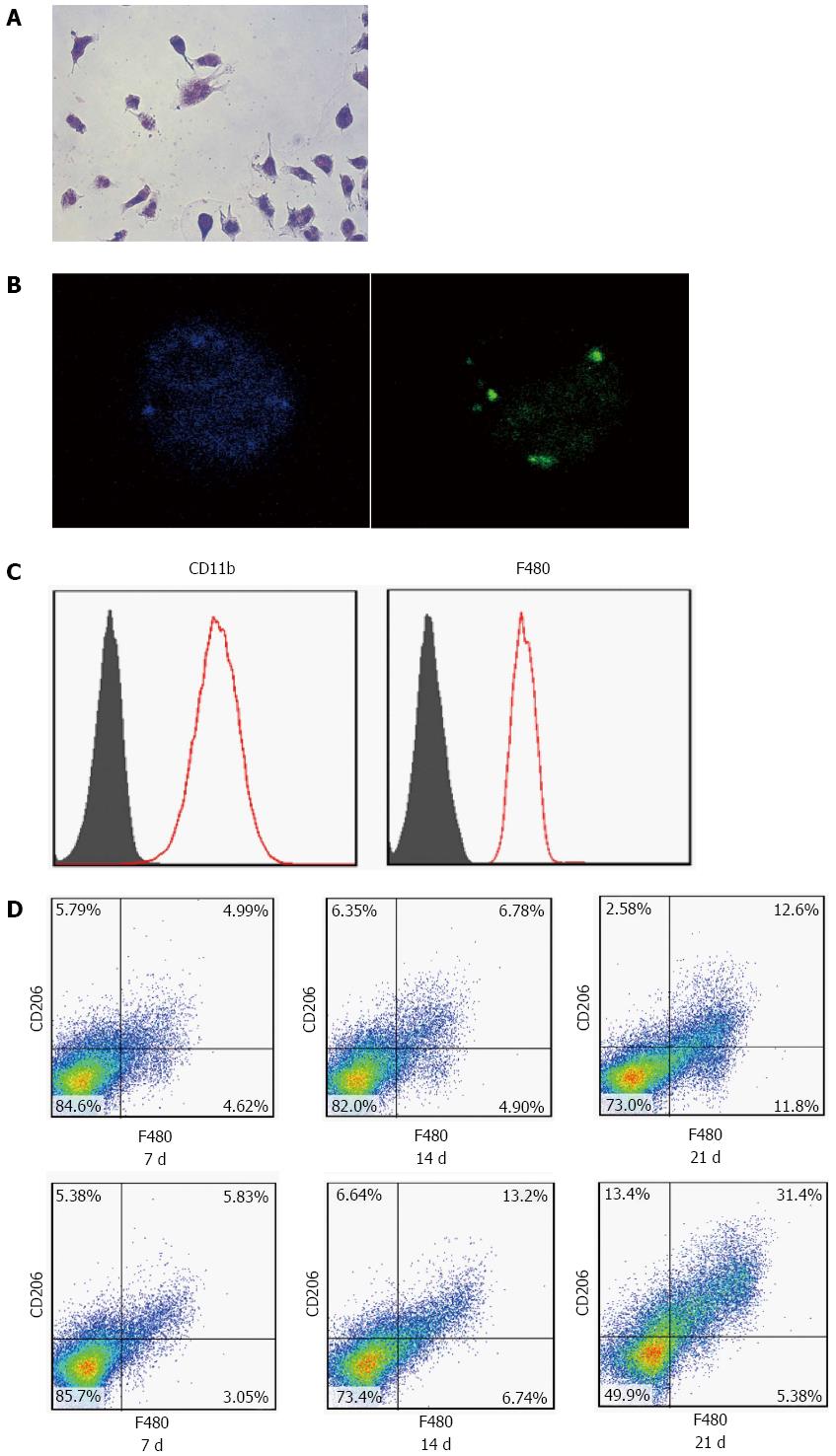
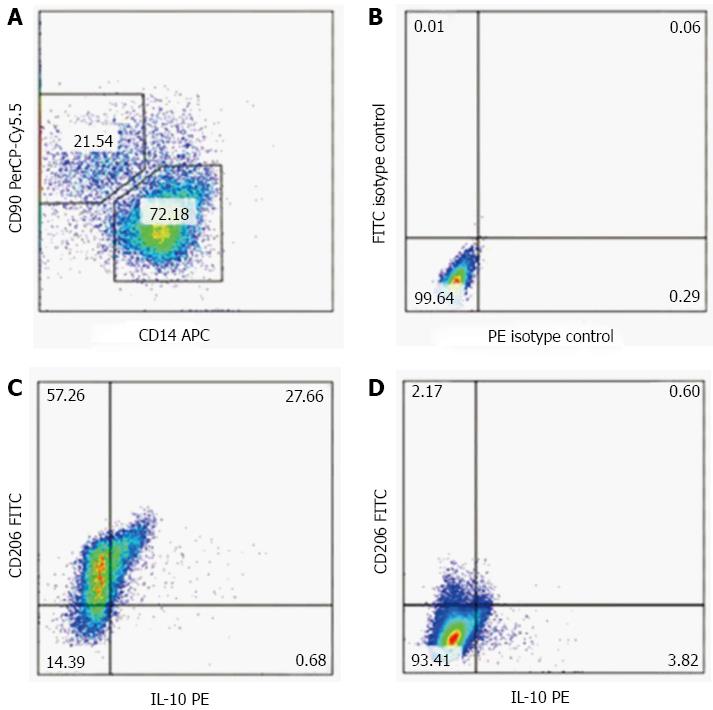
Figure 4 Umbilical cord-derived mesenchymal stem cells transfusion promotes polarization of Kupffer cells in the fibrotic liver.
Kupffer cells (KCs) were isolated from the liver. The morphology of cells cultured with FITC-labeled ovalbumin showed green fluorescent (A, B) cells that were CD11b- and F480-positive, as assessed by flow cytometry analysis (C). After transfusion with UC-MSCs, CD206-positive KCs increased significantly in a time-dependent manner (D).
Figure 5 Umbilical cord-derived mesenchymal stem cells promote polarization of KCs in vitro.
We isolated Kupffer cells (KCs) from rats treated with DMN for three consecutive weeks, and co-cultured them with umbilical cord-derived mesenchymal stem cells (UC-MSCs). KCs isolated from the same rats and cultured alone were set as the control group. Less than 4% of CD206 positive M2 cells appeared in KCs cultured alone; whereas, more than 80% M2 cells appeared in KCs co-cultured with UC-MSCs (A). Given the anti-fibrogenic effect of M2 cells, and the M1 cells’ pro-fibrogenic effect, our data showed that UC-MSCs promoted KC polarization in vitro.
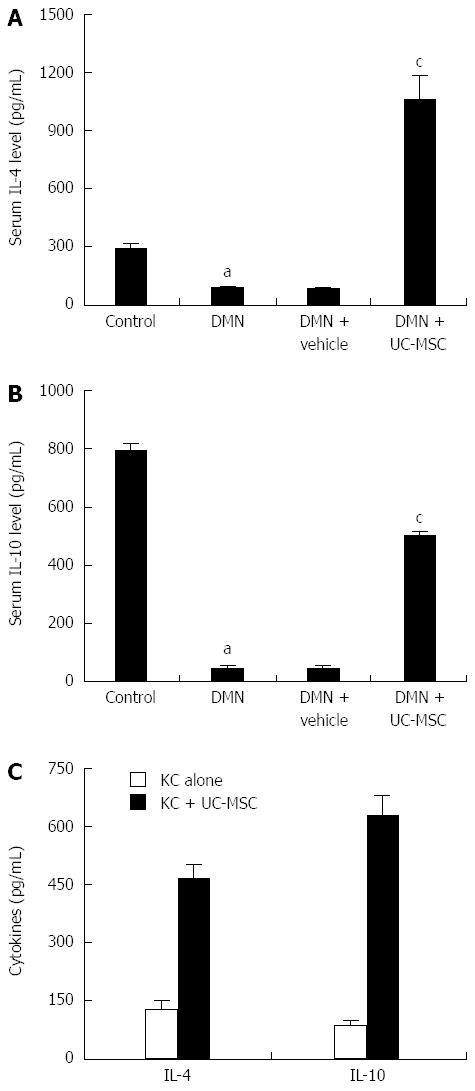
Figure 6 Umbilical cord-derived mesenchymal stem cells transfusion promotes the production of IL-4 and IL-10 in vivo and in vitro.
Serum IL-4 and IL-10 levels were detected by ELISA. IL-4 and IL-10 levels declined significantly after DMN treatment, and this effect was blocked by umbilical cord-derived mesenchymal stem cells (UC-MSCs) transfusion. Serum IL-4 and IL-10 levels were elevated significantly in DMN rats transfused with UC-MSCs compared with rats treated with DMN alone (A and B, aP < 0.01 compared with the control group; cP < 0.01 compared with the DMN + vehicle group). In vitro, we co-cultured UC-MSCs with Kupffer cells (KCs) isolated from DMN rats, and cell culture supernatants were prepared for ELISA: IL-4 and IL-10 levels in KC plus UC-MSCs were, respectively, more than 3- and 4-fold higher than the values obtained for KC cultured alone (C). The consistent results from in vivo and in vitro experiments indicated that UC-MSCs promote IL-4 and IL-10 production in KCs, which resulted in subsequent KC polarization.
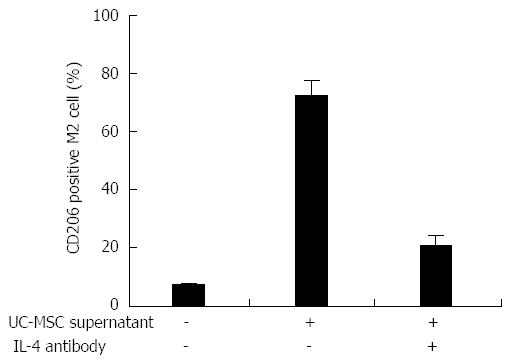
Figure 7 Umbilical cord-derived mesenchymal stem cells secrete IL-4 and promote Kupffer cells polarization in vitro.
We isolated Kupffer cells (KCs) from DMN rats. Flow cytometry analysis showed that about 6% of the KCs were CD206 positive M2 cells; when umbilical cord-derived mesenchymal stem cells (UC-MSCs) were added into the culture medium, M2 cells increased to more than 70%. Interestingly, anti-IL-4 antibody treatment blocked the UC-MSC effect and less than 30% KCs became M2 cells.
- Citation: Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol 2016; 22(26): 6036-6048
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6036