Copyright
©The Author(s) 2016.
World J Gastroenterol. Mar 28, 2016; 22(12): 3363-3371
Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3363
Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3363
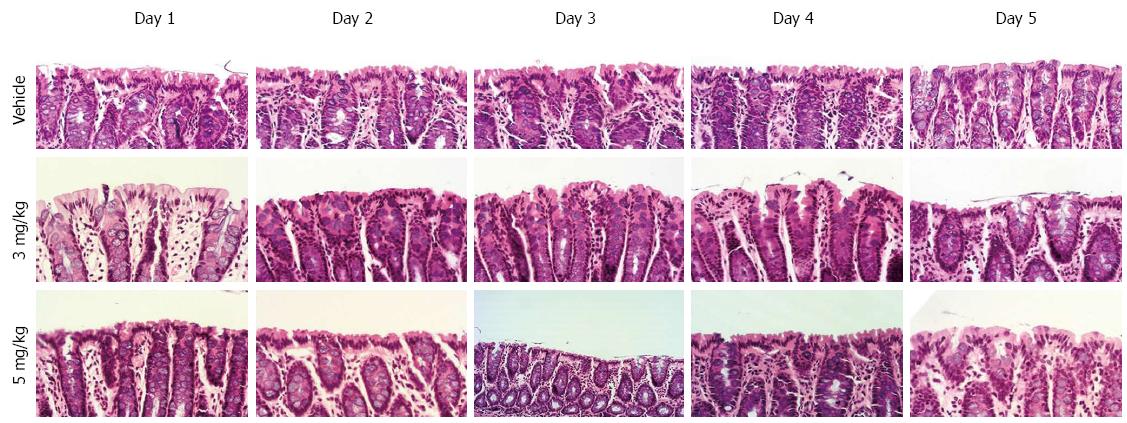
Figure 1 Hematoxylin and eosin staining of the distal colon of cirrhotic rats with ascites receiving different doses of tolvaptan for different periods (× 400).
No mucosal damage was attributable to administration of tolvaptan.
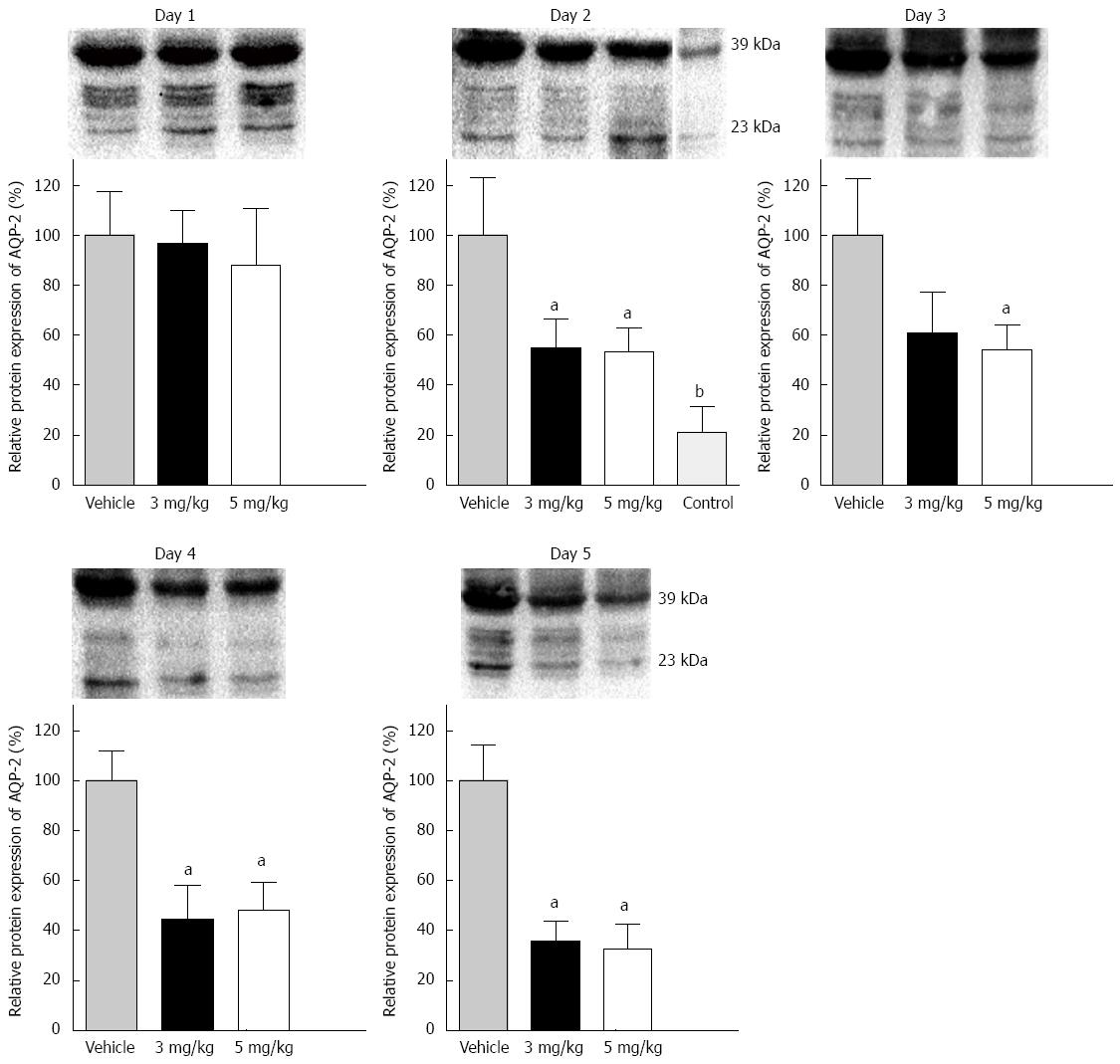
Figure 2 Phosphorylated (39 kDa) and nonphosphorylated (23 kDa) protein expression of AQP-2 in distal colon in cirrhotic rats with ascites treated by oral gavage of tolvaptan (3 or 5 mg/kg) or vehicle.
After 1 d of both doses of tolvaptan, no significant difference was found between each group. Compared with vehicle, AQP-2 protein expression showed significant reductions on day 2 (3 mg/kg: 100% ± 22.9% vs 54.7% ± 11.7%, P < 0.05; 5 mg/kg: 100% ± 22.9% vs 53.0% ± 9.4%, P < 0.01) and on successive days. AQP-2 expression in a control group (no treatment) was also measured, and was significantly lower than that on day 2 compared with vehicle (22.2% ± 10.23% vs 100% ± 22.9%, P < 0.01). No significant difference was found between the cirrhotic groups treated with different dosesof tolvaptan. Data are mean ± SEM; aP < 0.05, tolvaptan vs vehicle; bP < 0.01, tolvaptan vs vehicle. AQP: Aquaporin.
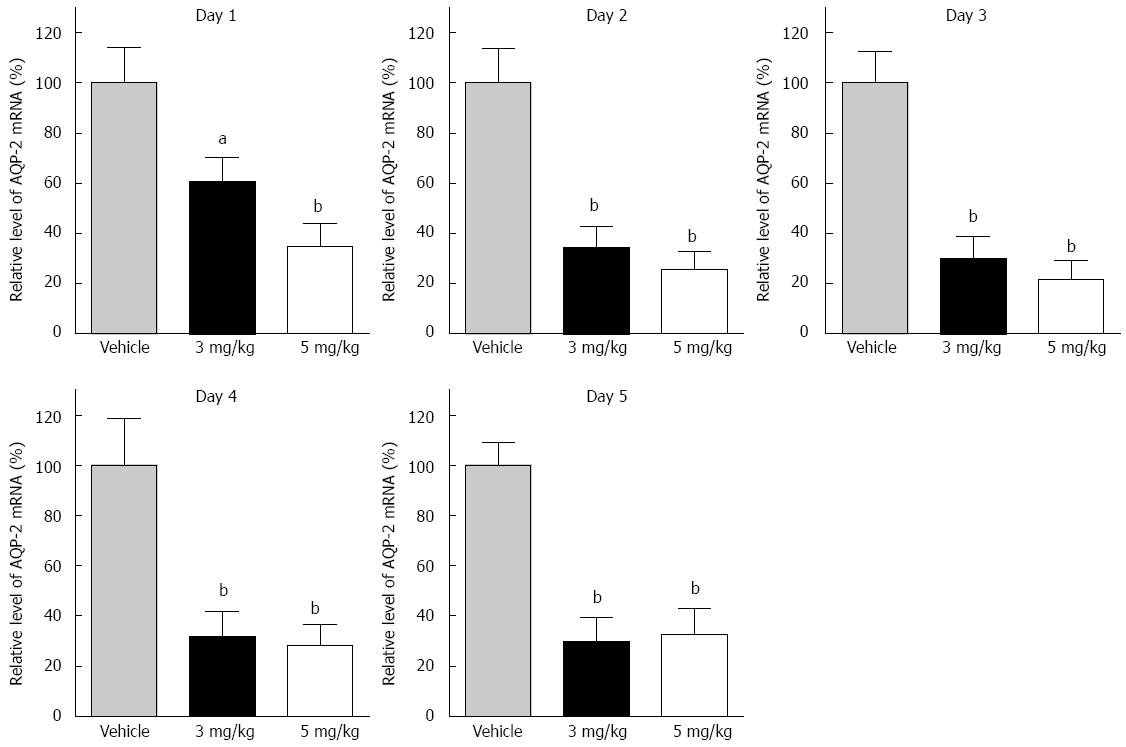
Figure 3 Relative mRNA expression of AQP-2 in the distal colon in the tolvaptan and vehicle groups.
AQP-2 transcription in the distal colon in cirrhotic rats with ascites treated with different doses of tolvaptan (3 and 5 mg/kg) was measured. Compared with vehicle, a significant difference was detected on day 1 (3 mg/kg: 100% ± 16.3% vs 68.5% ± 10.0%, P < 0.05; 5 mg/kg: 100% ± 16.3% vs 34.1% ± 15.1%, P < 0.01) and on successive days. There was no significant difference between the respective tolvaptan groups. Data are mean ± SEM; aP < 0.05, tolvaptan vs vehicle; bP < 0.01, tolvaptan vs vehicle.
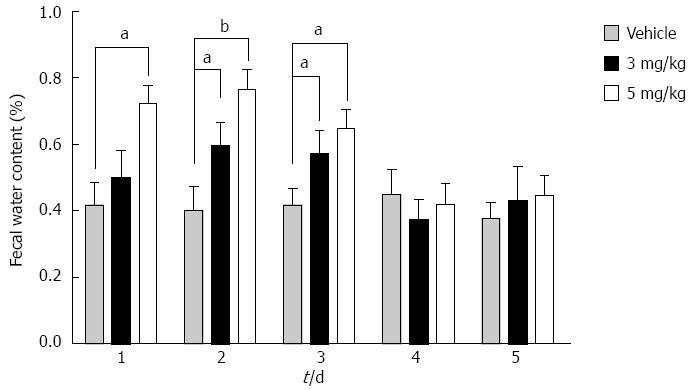
Figure 4 Fecal water content in the distal colon of cirrhotic rats with ascites.
Compared with vehicle, the fecal water content increased from day 1 in the 5 mg/kg tolvaptan group (41.4% ± 6.3% vs 66.8% ± 9.3%, P < 0.05), and from day 2 in the 3 mg/kg group (40.0% ± 6.0% vs 59.2% ± 10.3%, P < 0.05). However, it returned to baseline after 3 d administration of both doses of tolvaptan. No significant difference was found between different doses of tolvaptan. Data are mean ± SEM; aP < 0.05, tolvaptan vs vehicle; bP < 0.01, tolvaptan vs vehicle.
- Citation: Chen C, Chen RP, Lin HH, Zhang WY, Huang XL, Huang ZM. Tolvaptan regulates aquaporin-2 and fecal water in cirrhotic rats with ascites. World J Gastroenterol 2016; 22(12): 3363-3371
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3363.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3363