Copyright
©The Author(s) 2016.
World J Gastroenterol. Jan 7, 2016; 22(1): 50-71
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.50
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.50
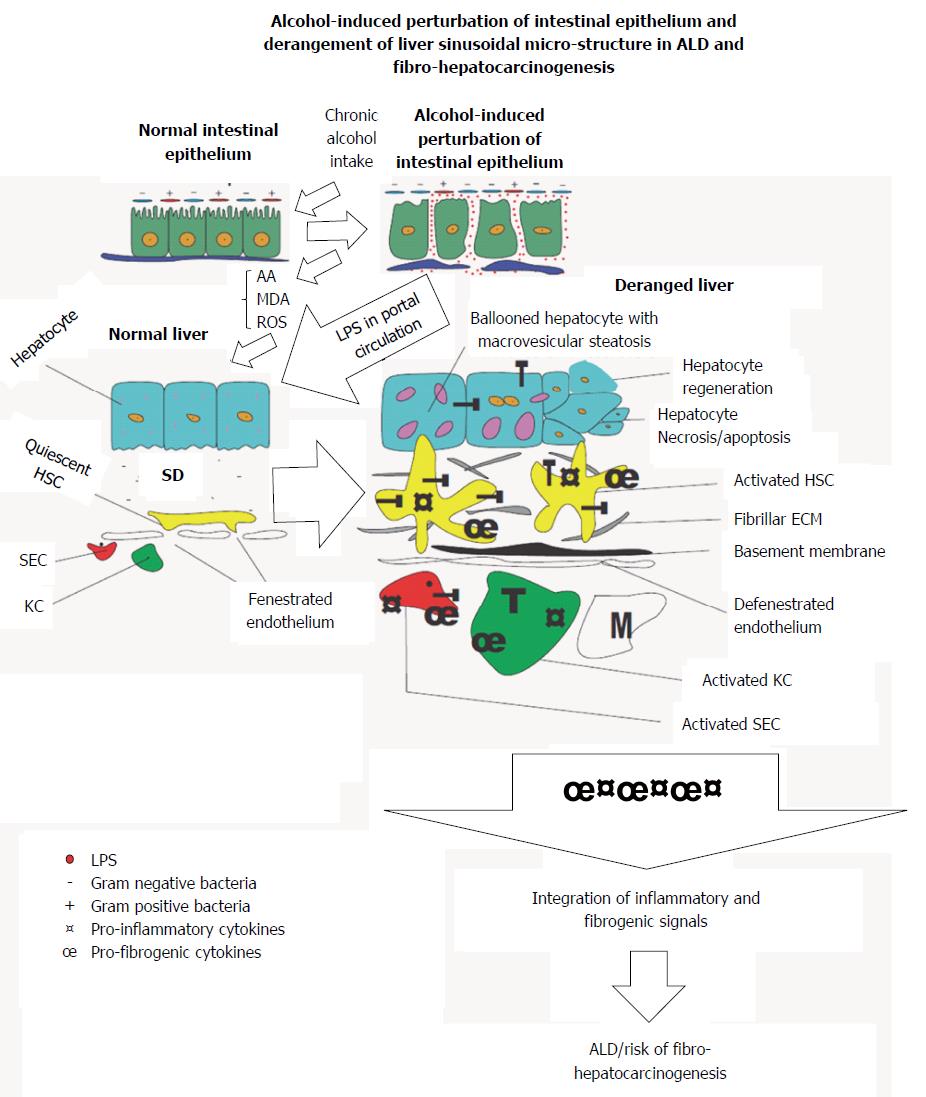
Figure 1 An illustration of the bidirectional origin of alcoholic liver disease and fibro-hepatocarcinogenesis within the gut-liver axis.
Chronic alcohol use induces derangement of the gut epithelium, increases Gram-/Gram+ bacteria ratio, increases endotoxin turnover, increases permeability of gut epithelium to endotoxins including lipopolysaccharide (LPS). Subsequently leakage of LPS into portal circulation gain access to liver to initiate activation of hepatic cells. LPS-dependent activation of hepatic cells is further augmented by metabolic derivatives of alcohol to promote alcoholic liver disease (ALD) and fibro-hepatocarcinogenesis. T: Toll-like receptor 4; HSC: Hepatic stellate cell; KC: Kupffer cell; SD: Space of disse; SEC: Sinusoidal endothelial cell; M: Monocyte.
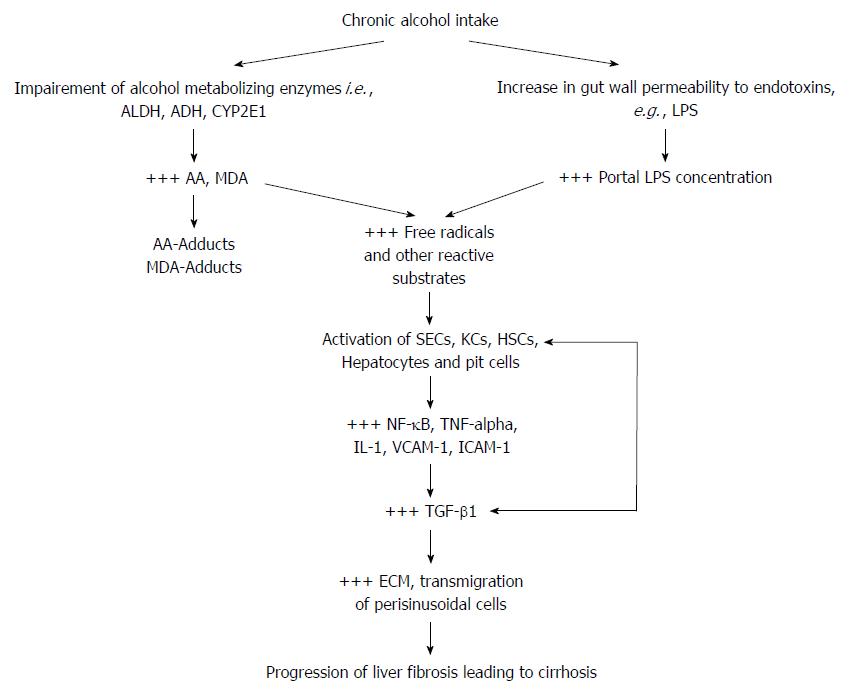
Figure 2 A schematic illustration of the two-way attack of hepatic cells in alcoholic liver disease and fibro-hepatocarcinogenesis.
By multiple mechanisms alcohol metabolizing enzymes and gut-derived LPS induce production of free radicals which stimulate the release of pro-inflammatory and pro-fibrogenic cytokines. Free radical-dependent activation of hepatic cells leads to the release of pro-inflammatory transcription factor (NF-κB) and inflammatory cytokines (TNF-α, IL-1β) which provide the signal for injurious overt hepatic inflammatory response. Secondary to the injurious hepatic inflammatory response, is the activation of hepatic cells (mainly HSCs and KCs) to further release pro-fibrogenic factors, mainly the key fibrogenic cytokine (TGF-β) which mediates a high ECM turnover (increased fibrogenesis : fibrinolysis ratio) in HSCs and the space of Disse, and trans-migration of hepatic non-parenchymal cells. These pathological events initiate liver fibrosis and cirrhosis leading to fibro-hepatocarcinogenesis. AA: Acetaldehyde; ADH: Aldehyde dehydrogenase; ALDH: Alcohol dehydrogenase; ECM: Extracellular matrix; HSCs: Hepatic stellate cells; IL-1β: Interleukin-1 beta; KCs: Kupffer cells; LPS: Lipopolysaccharide; MDA: Malondialdehyde; NF-κB: Nuclear factor kappa B; SECs: Sinusoidal endothelial cells; TGF-β: Transforming growth factor beta; TNF-α: Tumor necrosis factor alpha; VCAM: Vascular cell adhesion molecule; +++: Up-regulated expression or overproduction.
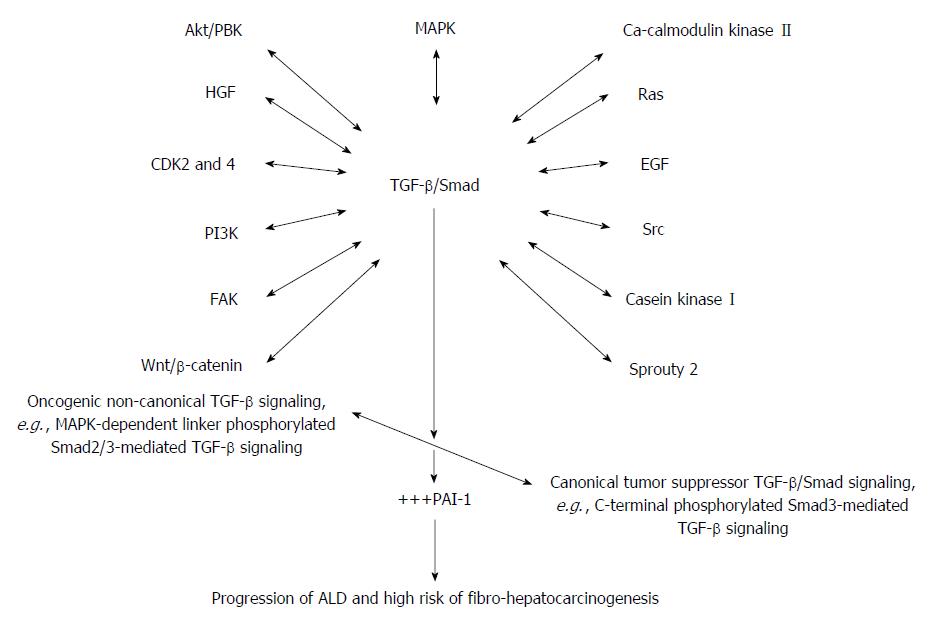
Figure 3 A proposed schematic illustration of the complex and integrative role of TGF-β/Smad signaling in alcoholic liver disease and alcohol-induced fibro-hepatocarcinogenesis.
Alcohol and its metabolic derivatives induce the release and activation of TGF-β/Smad signaling through NF-κB/TNF-α mediation (Figure 1). The NF-κB/TNF-α-mediated activation of TGF-β/Smad signaling switches canonical tumor suppressor (C-terminal phosphorylated Smad3/Smad4-dependent TGF-β signaling) into oncogenic (MAPK-dependent linker phosphorylated Smad2/3-dependent TGF-β signaling) and also non-canonical TGF-β signaling involving cross-signaling with other signaling pathways implicated in hepatic malignancies. Key cross-signaling pathways which team up with TGF-β signaling includes but not limited to CDK 2 and 4, Ca-calmodulin kinase II, EGF, HGF, PI3K/AKT, FAK, Src, Sprouty2, casein kinase I, Wnt/β-catenin. This leads to imbalance between canonical and non-canonical TGF-β signaling. Increase in oncogenic TGF-β/Smad signaling leads to up-regulation of PAI-1 gene expression and PAI-1-mediated pathologies thereof. The key pathological effects of PAI-1 include dysregulated ECM regulation, cell proliferation and invasion, and dysregulated apoptosis and these underlie initiation and progression of alcohol-induced fibro-hepatocarcinogenesis. HGF: Hepatocyte growth factor; ECM: Extracellular matrix; PAI: Plasminogen activator inhibitor; PI3K: Phosphatidylinositol 3-kinase; EGF: Epidermal growth factor; MAPK: Mitogen activated protein kinase; TGF-β: Transforming growth factor beta; Smad: Small mother against decapentaplegic; +++: Up-regulated expression or overproduction.
- Citation: Boye A, Zou YH, Yang Y. Metabolic derivatives of alcohol and the molecular culprits of fibro-hepatocarcinogenesis: Allies or enemies? World J Gastroenterol 2016; 22(1): 50-71
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/50.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.50