Copyright
©The Author(s) 2016.
World J Gastroenterol. Jan 7, 2016; 22(1): 338-348
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.338
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.338
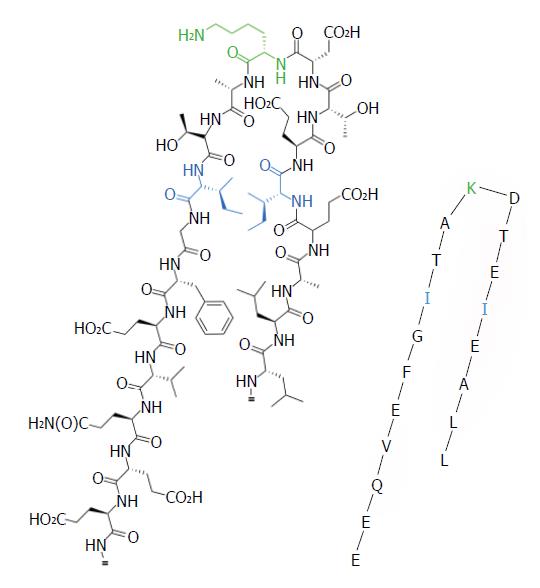
Figure 1 Schematic representation of the pyruvate dehydrogenase E2 lipoyl domain.
Including the 19 residues (LLAEI-ETDKA-TIGFE-VQEE), lipoic acid is covalently attached to the ε group of lysine (K) via an amide bond.
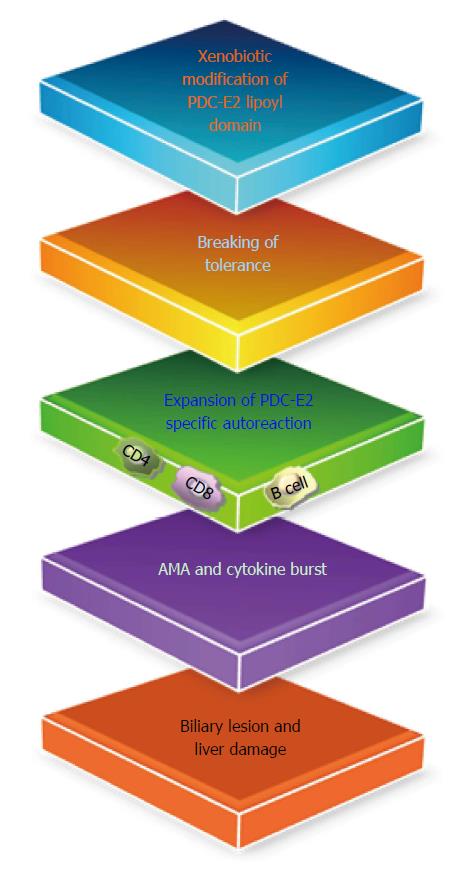
Figure 2 Xenobiotic modification of the native lipoyl moiety of the major mitochondrial autoantigen pyruvate dehydrogenase E2, lead to the loss of self-tolerance and eventually biliary lesions in primary biliary cholangitis.
PDC-E2: Pyruvate dehydrogenase E2; AMA: Anti-mitochondrial autoantibody.
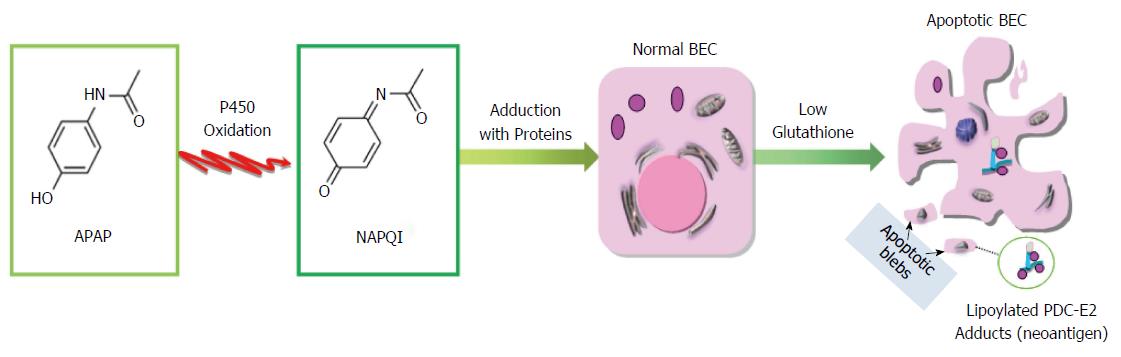
Figure 3 APAP metabolism and proposed mechanism of APAP-mediated breaking of immune tolerance.
APAP is metabolized in the liver to nontoxic compounds via conjugation of the aromatic ring to sulfate or glucuronic acid. APAP is converted into a highly electrophilic metabolite, NAPQI by microsomal cytochrome P450 oxidation. Reactive NAPQI accumulates and can form adducts with cellular proteins, leading to disruption of cellular functions, generation of neo-antigens, and loss of tolerance.
- Citation: Wang J, Yang G, Dubrovsky AM, Choi J, Leung PS. Xenobiotics and loss of tolerance in primary biliary cholangitis. World J Gastroenterol 2016; 22(1): 338-348
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.338