Copyright
©The Author(s) 2016.
World J Gastroenterol. Jan 7, 2016; 22(1): 300-325
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.300
Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.300
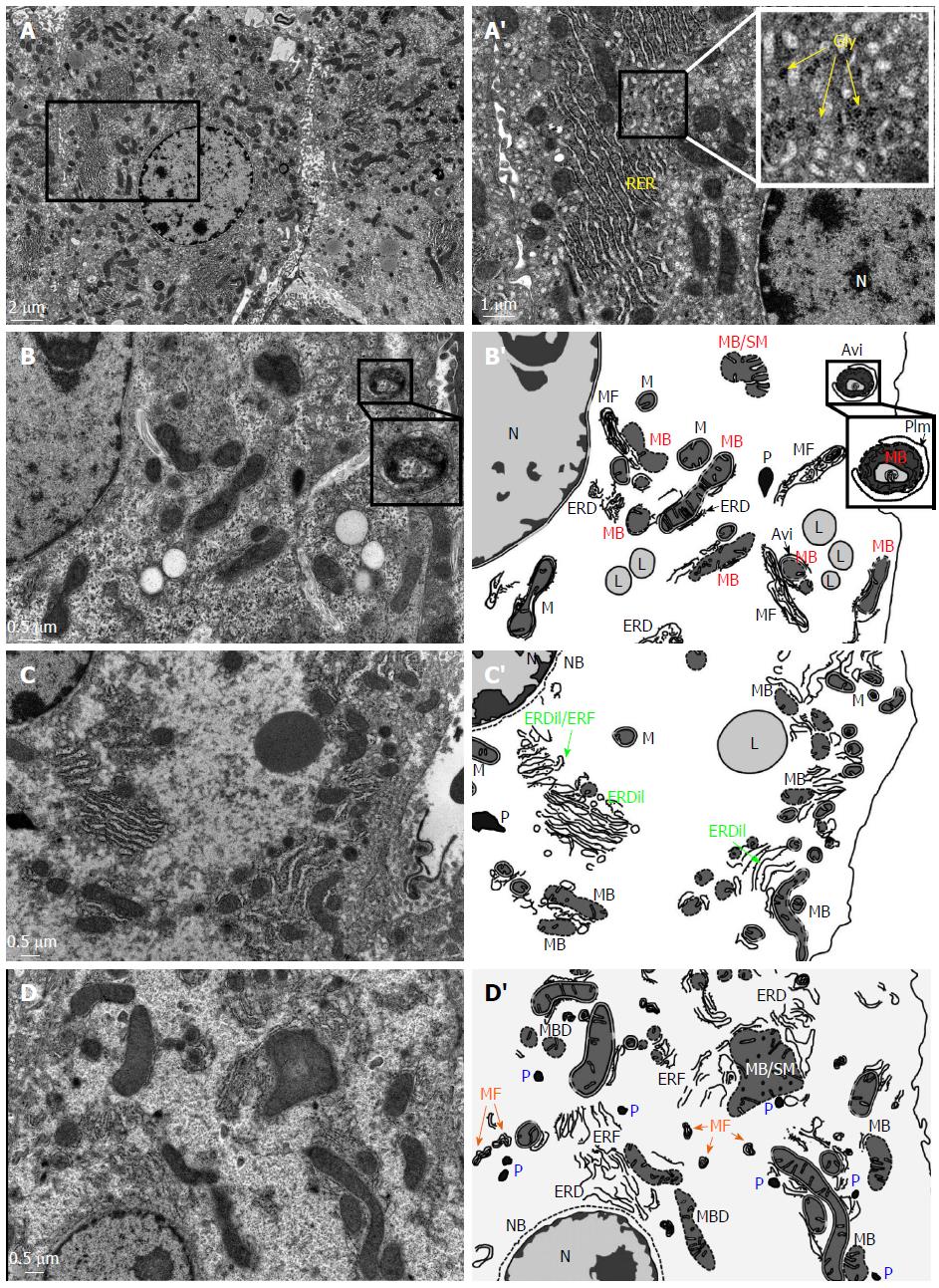
Figure 1 Ultrastructural alterations in the hepatocytes of the hepatitis B virus X protein transgenic mice revealed by transmission electron microscope.
A: Ultrastructure of the hepatocyte of a wild-type mouse (2-mo old). A’: the inset in A was enlarged to provide a better perspective of intact mitochondria (M), rough endoplasmic reticulum (RER), rosette glycogen (Gly) and nucleus (N). B-D: In the HBx transgenic mice (2-mo old), severe ultrastructural alterations can be observed in the hepatocytes. These include nuclear envelope breakdown (NB), a significant decrease in the density of cytoplasmic organelles, abundant peroxisomes (P), the disorganization of rough endoplasmic reticulum [e.g., endoplasmic reticulum fragmentation (ERF), endoplasmic reticulum dilation (ERDil), and/or endoplasmic reticulum degeneration ERD)], mitochondria membrane breakdown (MB), swollen mitochondria (SM), the presence of lipid droplets (L), and appearance of myelin figures (MF), which are the membranous debris of mitochondria or ER degeneration. B’-D’: Schematic presentation of the ultrastructural alterations in the HBx hepatocytes shown in panel B-D. The inset in B and B’ provides a better perspective of a phagophore isolation membrane (PIm) engulfing a degenerated mitochondrion leading to the formation of autophagosomes (AVi).
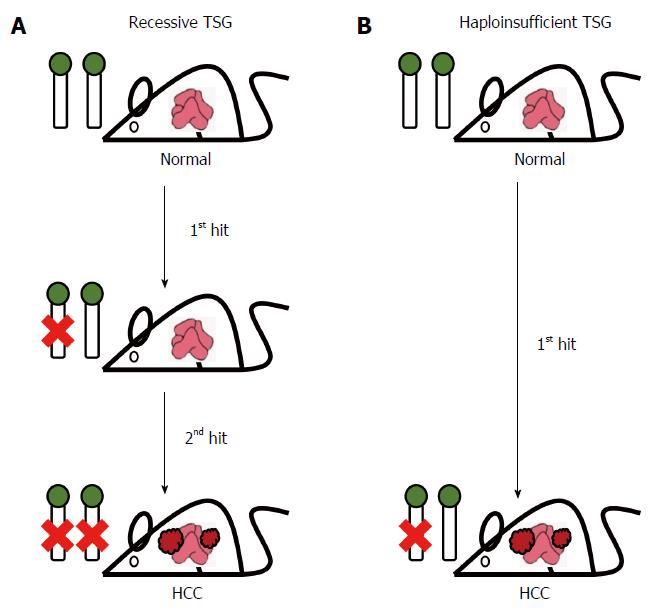
Figure 2 Tumor suppressor genes in hepatocellular carcinoma.
A: Recessive tumor suppressor genes (TSGs) need there to be loss-of-function via two hits (mutation or genetic modification) in order to trigger tumor formation. Only when there is homozygous deficiency of a recessive TSG does this lead to hepatocellular carcinoma (HCC) development; B: Haploinsufficient TSGs are functionally insufficient to suppress tumor development when there is loss of only a single allele. Heterozygous deficiency of a haploinsufficient TSG can cause HCC development in the liver even when the second allele remains intact.
- Citation: Teng YC, Shen ZQ, Kao CH, Tsai TF. Hepatocellular carcinoma mouse models: Hepatitis B virus-associated hepatocarcinogenesis and haploinsufficient tumor suppressor genes. World J Gastroenterol 2016; 22(1): 300-325
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/300.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.300