Copyright
©The Author(s) 2015.
World J Gastroenterol. Oct 28, 2015; 21(40): 11396-11410
Published online Oct 28, 2015. doi: 10.3748/wjg.v21.i40.11396
Published online Oct 28, 2015. doi: 10.3748/wjg.v21.i40.11396
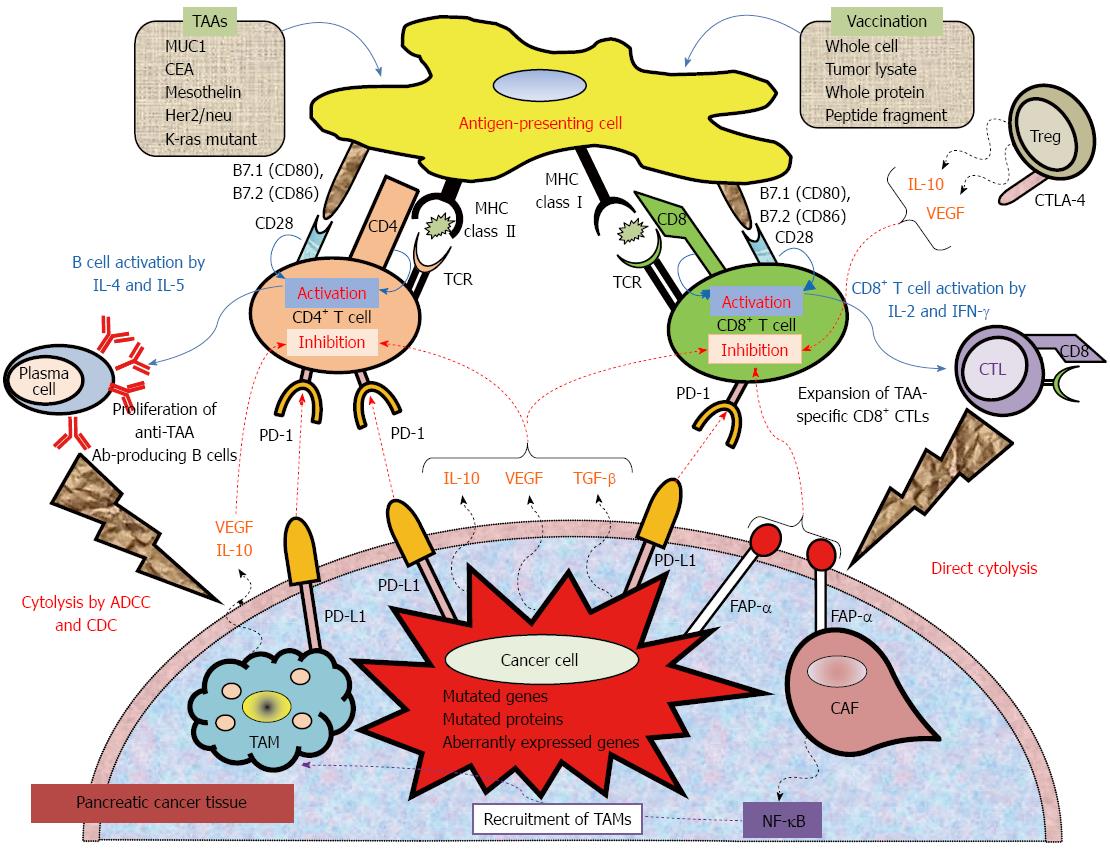
Figure 1 Mechanistic basis for vaccine-based immunotherapy, and immune co-stimulatory, co-inhibitory ligands and receptors, and soluble immune modulating factors involved in T cell activation and inhibition.
Anticancer immunotherapy aims to harness the natural ability of the immune system to recognize and react against potential TAAs. Peptide-based, protein-based, or whole cell-based vaccines rely on identified immune-dominant TAA epitopes to stimulate anticancer T-cell responses. Antigen-presenting cells (APCs), including dendritic cells, capture antigens obtained from vaccinations. After intracellular processing, antigen peptides are loaded onto major histocompatibility complex (MHC class I or II) molecules on the surface of the APC. Specific T cells encounter these MHC-peptide complexes in conjugation with a co-stimulatory signal. The activated T cells proliferate and secrete cytokines (IL-2, IL-4, IL-5, INF-γ), resulting in the production of a cascade of immune effector cells. Immunotherapeutic strategies that inhibit immune checkpoints such as those mediated by CTLA-4 and PD-1 reduce the barriers that vaccines must overcome to trigger therapeutically relevant anticancer immune responses. Recently, preclinical and clinical studies have demonstrated that combining immune-modulating agents such as cyclophosphamide (CY) and checkpoint inhibitors (anti-CTLA-4, anti-PD-1, anti-PD-L1) with vaccine strategies can enhance anticancer immune responses as well as block the tolerizing mechanisms that would otherwise inhibit these responses. ADCC: Antibody-dependent cell-mediated cytotoxicity; CAF: Cancer-associated fibroblast; CDC: Complement-dependent cytolysis; CTLA-4: Cytotoxic T lymphocyte antigen-4; FAP-α: Fibroblast activation protein-α; TAM: Tumor-associated macrophage; TCR: T cell receptor; PD-1: Programmed cell death protein 1; PD-L1: Programmed death-ligand.
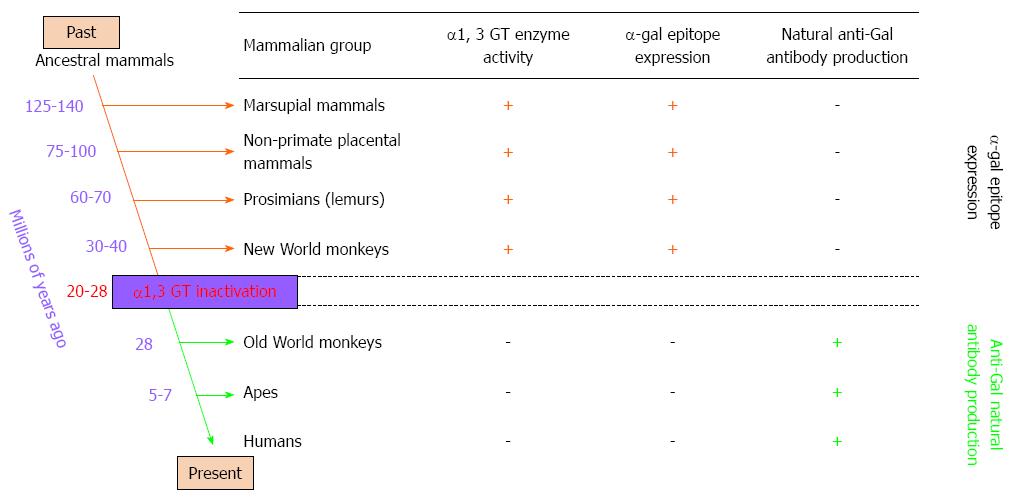
Figure 2 Reciprocal evolution of α1, 3 galactosyltransferase (α1, 3 GT) enzyme activity, α-gal epitopes, and anti-Gal antibody in mammals.
α-gal epitopes have been synthesized in mammals by α1, 3 galactosyltransferase (α1, 3 GT) for more than 125 million years, since before the divergence of placental mammals and marsupials. All non-mammalian vertebrates lack α1, 3 GT and do not express α-gal epitopes. Expression of this epitope was suppressed in ancestral Old World primates after they diverged from New World monkeys, and probably after apes and monkeys diverged from each other. Suppression of α-gal epitopes was followed by production of natural anti-Gal antibody, which is absent in non-primate mammals, prosimians, and New World monkeys.
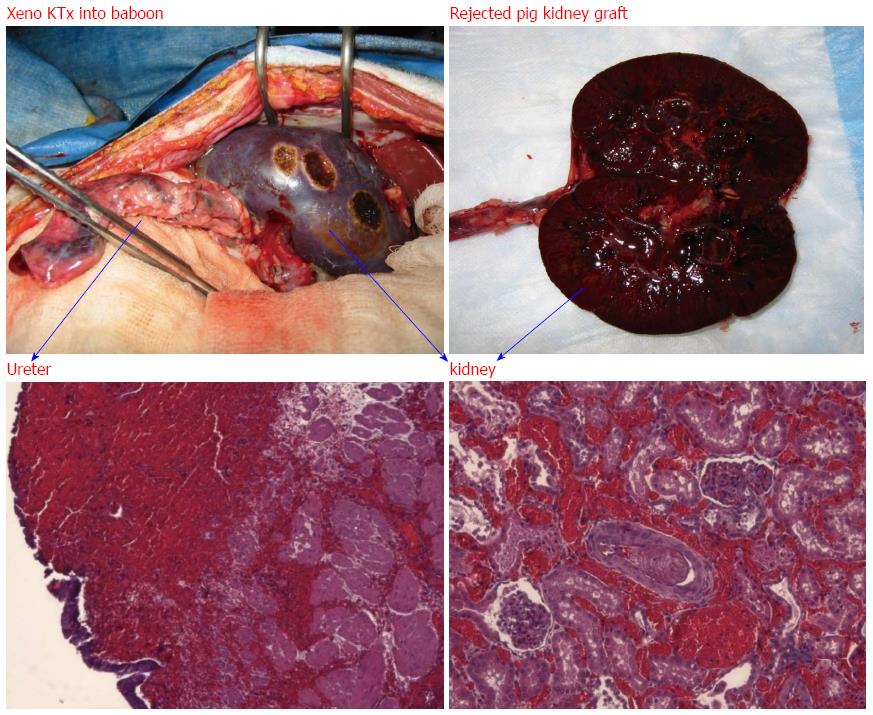
Figure 3 Hyperacute rejection of α-gal+/+ pig kidney xenografted into a baboon (1 d after kidney transplantation).
The interaction of natural baboon anti-Gal antibody with millions of α-gal epitopes expressed on the pig cell surface causes strong xenograft rejection. The in vivo binding of anti-Gal antibody to α-gal epitopes on transplanted pig heart or kidney is the main cause of hyperacute rejection of such grafts in humans and Old World monkeys. The recent generation of α1, 3 GT knockout pigs that lack α-gal epitopes has resulted in the elimination of this immunological barrier.
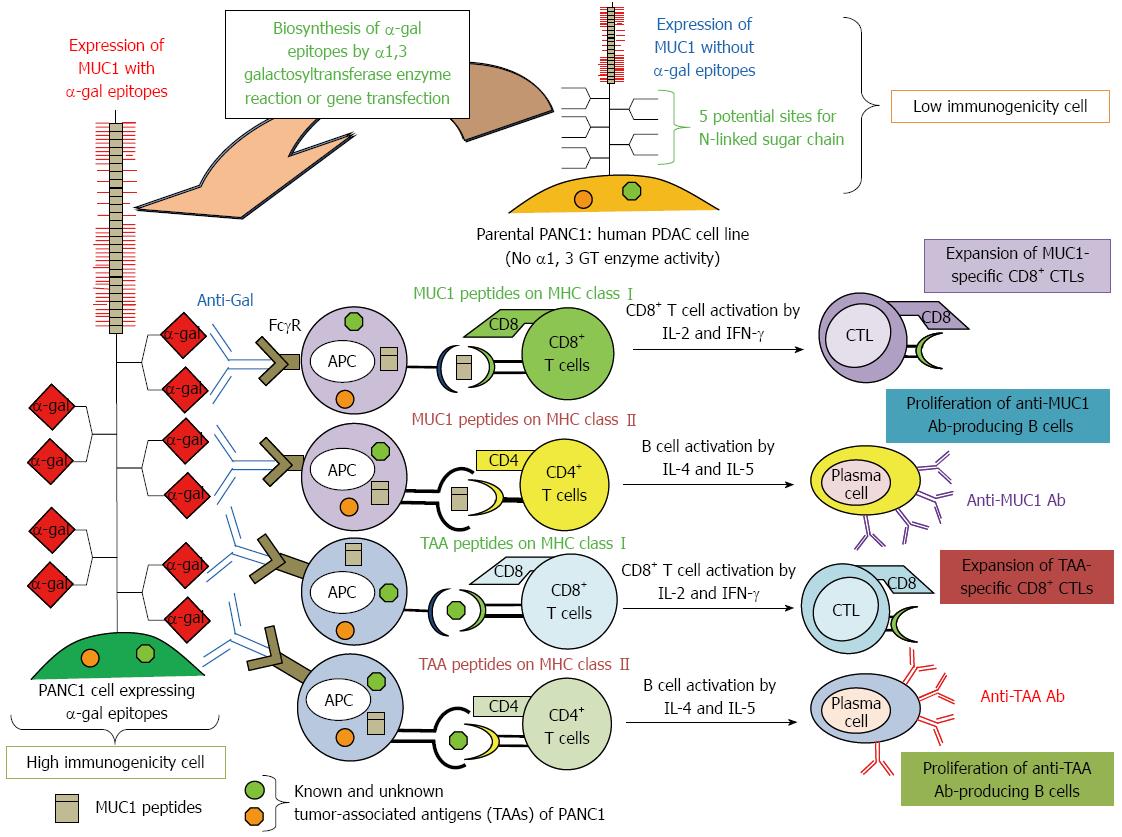
Figure 4 Increased immunogenicity of known and unknown tumor-associated antigens and MUC1 engineered to express α-gal epitopes.
Immunity towards known and unknown tumor-associated antigens (TAAs), including MUC1, in PDAC patients is relatively weak, and presentation of these TAAs to the immune system is poor due low immunogenicity. We tested the effects of vaccination using immunogenetically enhanced known and unknown TAAs and MUC1 with expression of α-gal epitopes on production of antibodies for MUC1 and other TAAs derived from PDAC cells, as well as induction of tumor-specific T cell activation.
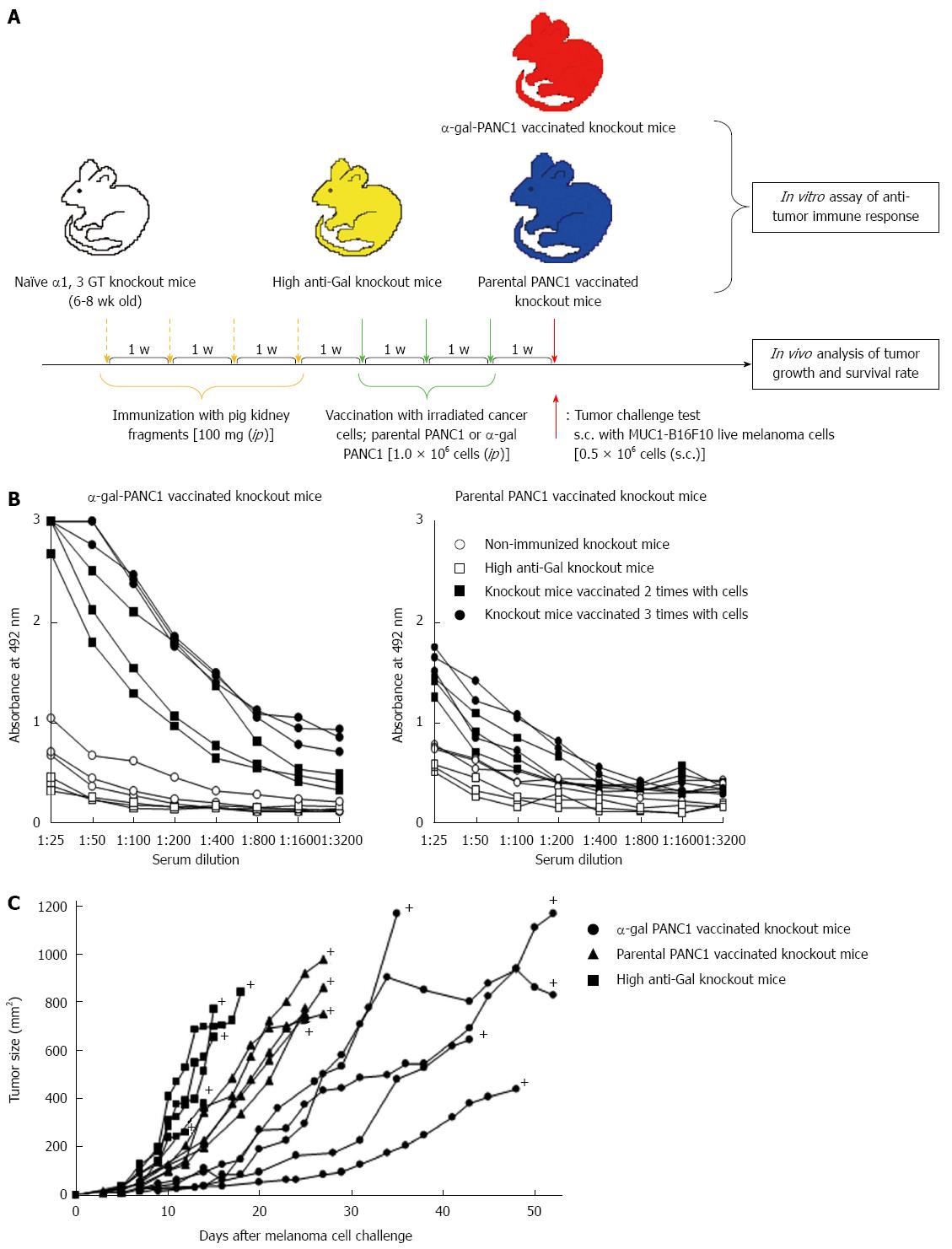
Figure 5 Experimental design for in vitro and in vivo studies and anti-MUC1 IgG antibody production assessed with an enzyme-linked immunosorbent assay.
A: Schematic illustration of the experimental protocol; B: Anti-MUC1 IgG production in knockout mice vaccinated with α-gal PANC1, and anti-MUC1 IgG production in knockout mice vaccinated with parental PANC1; C: Size of subcutaneous tumors after challenge with MUC1-B16F10 cells. +: Death.
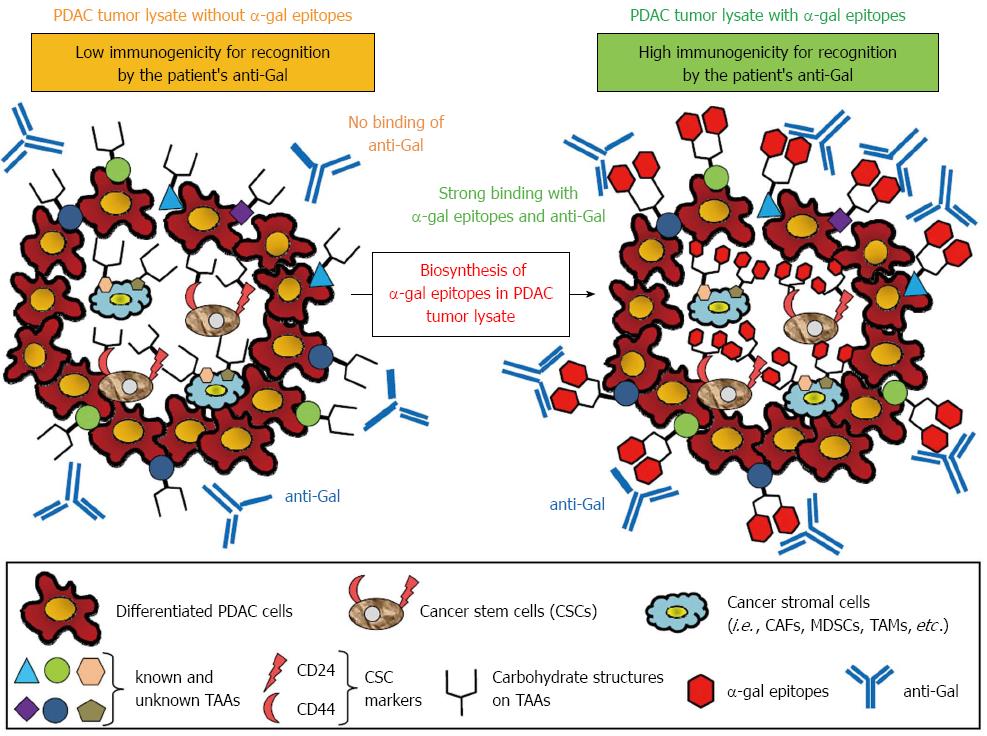
Figure 6 Concept of effective vaccination with α-gal tumor lysate against pancreatic ductal adenocarcinoma.
A tumor lysate is a more suitable source of tumor-associated antigens (TAAs) because it contains several known and unknown antigens in cancer cells and stromal cells that can elicit a broad-spectrum anti-tumor immune response. Moreover, the primary tumor of pancreatic adenocarcinoma contains a subset of pancreatic cancer cells with stem cell properties (i.e., pancreatic cancer stem cells: pancreatic CSCs). To increase the immunogenicity of known and unknown TAAs, CSC markers, or TAAs contained in cancer stromal cells to antigen-presenting cells, anti-Gal bound to α-gal-expressing TAAs could be a suitable strategy.
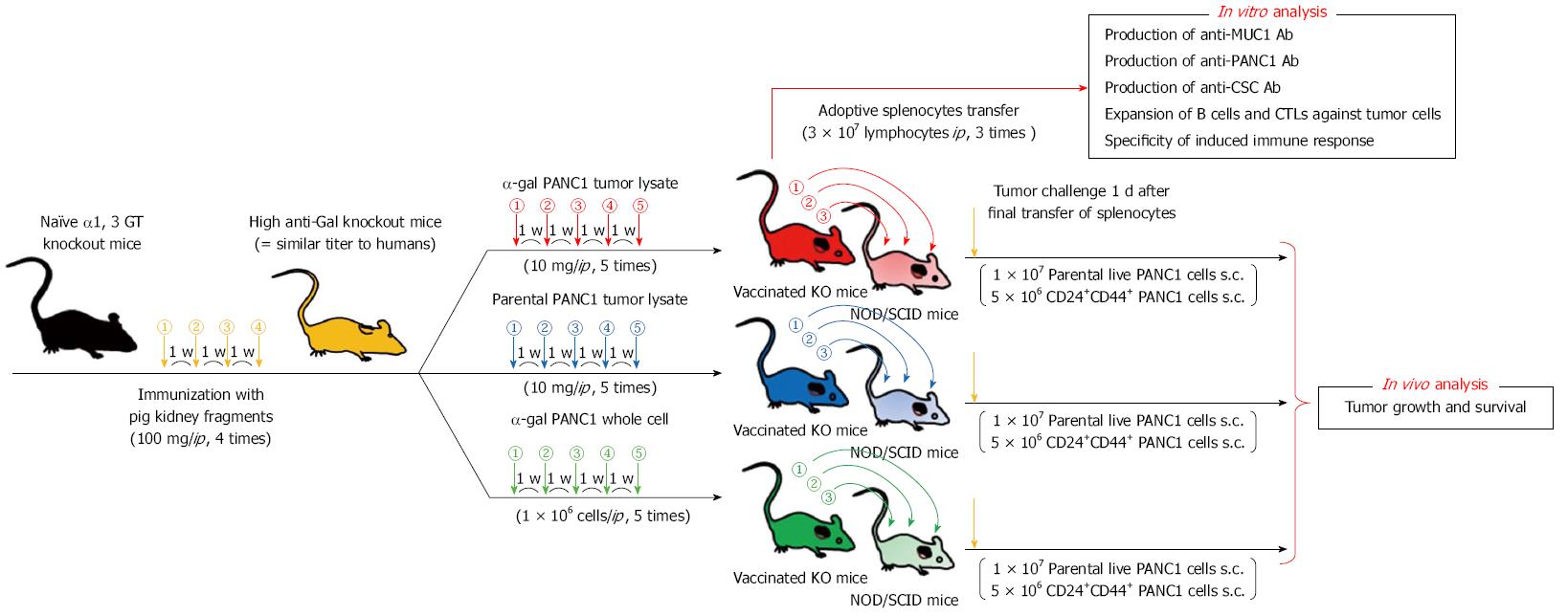
Figure 7 Experimental design of the adoptive transfer model using non-obese diabetic/severe combined immunodeficiency mice.
Experimental design of in vitro and in vivo studies was shown. For in vivo study, the adaptive transfer model, using non-obese diabetic/severe combined immunodeficiency mice as donors was employed.
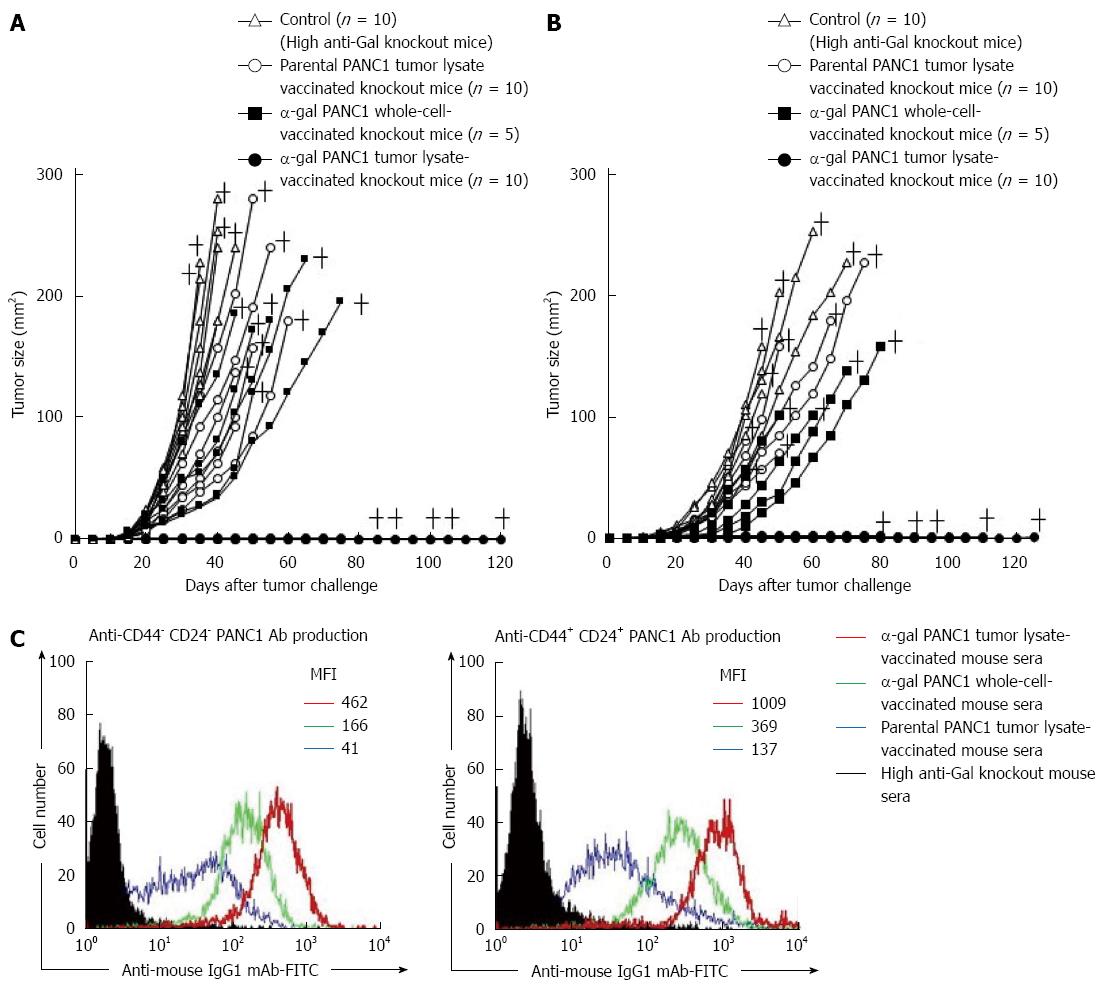
Figure 8 In vivo tumor growth in adoptively transferred non-obese diabetic/severe combined immunodeficiency mice challenged with either live PANC1 cells or live CD44+CD24+ PANC1 cells, and production of antibodies against differentiated cancer cells and cancer stem cells.
A: We monitored tumor growth in splenocyte-transferred mice. No tumors were noted in the α-gal tumor lysate-vaccinated mice. No significant differences in the time to appearance of a palpable tumor after tumor challenge were observed in the untreated control group and parental tumor lysate group (untreated: 10.6 ± 2.5 d; parental tumor lysate: 11.9 ± 2.1 d). In contrast, the development of tumors in the α-gal whole cell vaccination group was significantly delayed compared with the untreated and parental tumor lysate groups (α-gal whole-cell: 16.0 ± 2.8 d, P = 0.018 vs control; P = 0.004 vs parental tumor lysate). In the untreated control group, the maximum tumor size was 100 mm2 within 29 to 34 d (mean: 31.4 ± 2.1 d). In comparison, tumor growth to a similar size was markedly delayed in both the parental tumor lysate group (40.3 ± 6.9 d, P = 0.007 vs control) and α-gal whole-cell group (45.6 ± 8.3 d, P = 0.0013 vs control). +; death; B: The tumorigenesis of pancreatic CSCs was completely prevented in all α-gal tumor lysate-vaccinated mice. With the exception of the α-gal tumor lysate group, no significant differences were seen in the time to appearance of palpable tumors after tumor challenge among the groups (untreated: 13.1 ± 3.3 d; parental tumor lysate: 14.4 ± 3.4 d; α-gal whole-cell: 17.0 ± 3.8 d). The tumor size reached 100 mm2 in 40.6 ± 1.8 and 48.0 ± 4.4 d in the untreated and parental tumor lysate groups, respectively. However, tumor growth to a similar size was significantly delayed in the α-gal whole-cell group (60.5 ± 7.9 d; P < 0.001 vs control; P = 0.033 vs parental tumor lysate). +; death; C: Production of either anti-CD44-CD24- PANC1 (i.e., differentiated pancreatic cancer cells) antibodies or anti-CD44+CD24+ PANC1 (i.e., pancreatic cancer stem cells) antibodies in sera of vaccinated knockout mice assessed with flow cytometry. Closed histogram; stained cells with sera from non-vaccinated knockout mice, open histogram; stained cells with sera from vaccinated knockout mice. MFI: Mean fluorescence intensity.
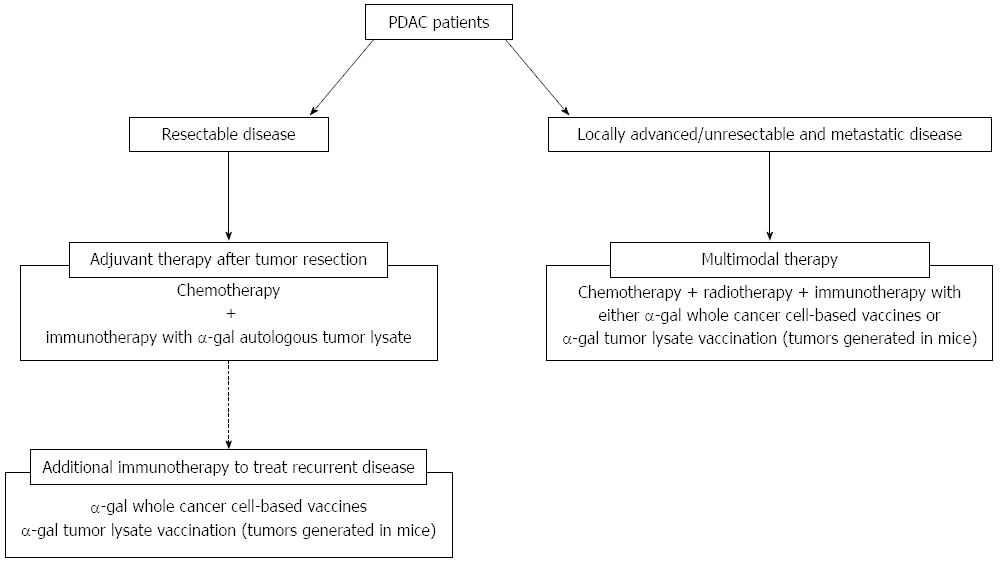
Figure 9 Treatment strategy using cancer immunotherapy utilizing α-gal epitope/anti-Gal antibody reaction for pancreatic ductal adenocarcinoma patients.
The clinical implications of this cancer immunotherapy model are shown. For patients with resectable disease, we plan to employ autologous tumor lysates prepared from surgically resected PDAC that is enzymatically engineered to express α-gal epitopes. For patients with recurrent disease after surgery, additional immunotherapy with either α-gal whole cancer cell-based vaccines or α-gal tumor lysate vaccination (tumors generated in mice) should be assessed. For patients with unresectable and metastatic disease, multimodal therapy, including cancer immunotherapy using either α-gal whole cancer cell-based vaccines or α-gal tumor lysate vaccination (tumors generated in mice) should be conducted.
- Citation: Tanemura M, Miyoshi E, Nagano H, Eguchi H, Matsunami K, Taniyama K, Hatanaka N, Akamatsu H, Mori M, Doki Y. Cancer immunotherapy for pancreatic cancer utilizing α-gal epitope/natural anti-Gal antibody reaction. World J Gastroenterol 2015; 21(40): 11396-11410
- URL: https://www.wjgnet.com/1007-9327/full/v21/i40/11396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i40.11396