Copyright
©The Author(s) 2015.
World J Gastroenterol. Oct 14, 2015; 21(38): 10840-10852
Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10840
Published online Oct 14, 2015. doi: 10.3748/wjg.v21.i38.10840
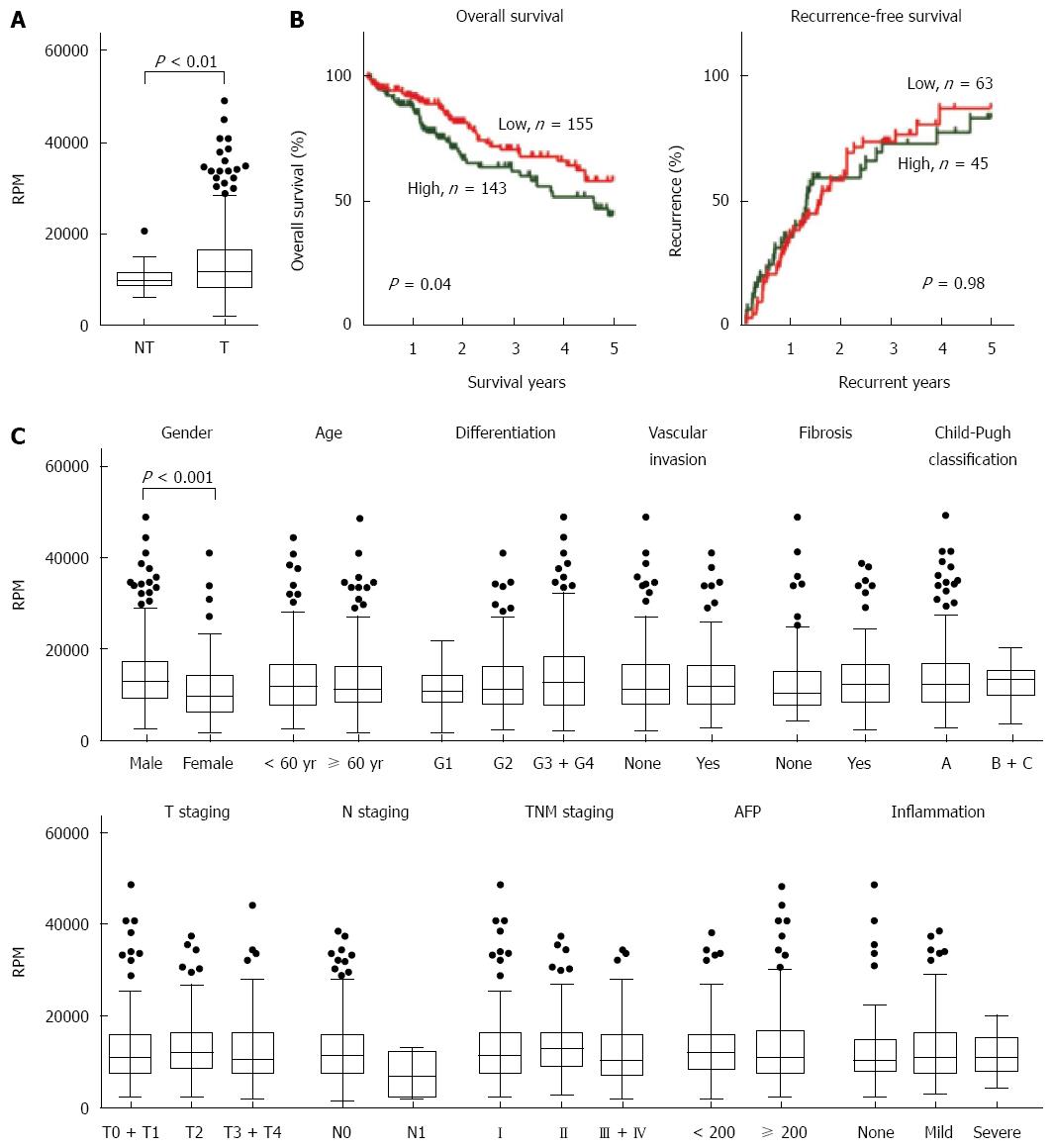
Figure 1 Expression and clinicopathological characteristics of peroxiredoxin 1 mRNA presented in The Cancer Genome Atlas liver cancer RNA sequencing dataset.
A: Peroxiredoxin 1 (PRDX1) mRNA was significantly up-regulated in tumor tissues (n = 374) compared with the adjacent non-tumor tissues (n = 50); B: Kaplan-Meier curves of overall survival (left panel) and recurrence (right panel) according to the PRDX1 levels in tumor samples. Log-rank test was performed; C: The clinicopathological characteristics analysis of PRDX1 expression in 374 liver cancer cases. RPM: Read per Million; RPM: Read per Million; AFP: α-fetoprotein.
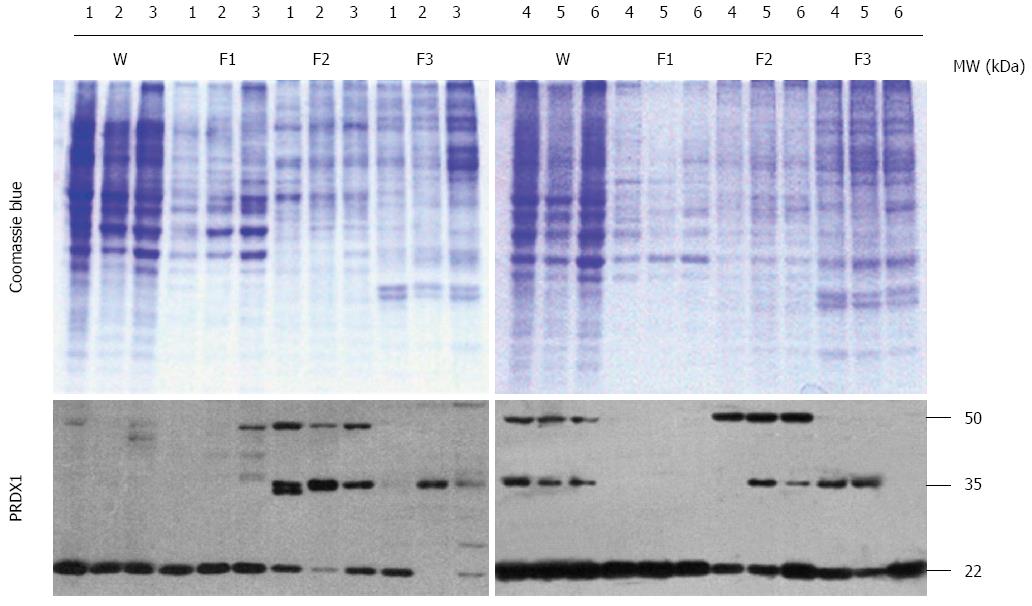
Figure 2 Western blotting analysis of peroxiredoxin 1 protein in liver cancer cells.
The codes of liver cancer cells: 1: HepG2; 2: Hep3B; 3: SK-HEP-1; 4: Bel-7404; 5: SMMC-7721; 6: HLE. W: Whole lysates; F1: Cytosol fraction; F2: Membrane/organelle fraction; F3: Nucleus fraction. The upper panel is the Coomassie Blue stained SDS-PAGE gel, and the lower panel is the Western blotting of PRDX1. PRDX1: Peroxiredoxin 1.
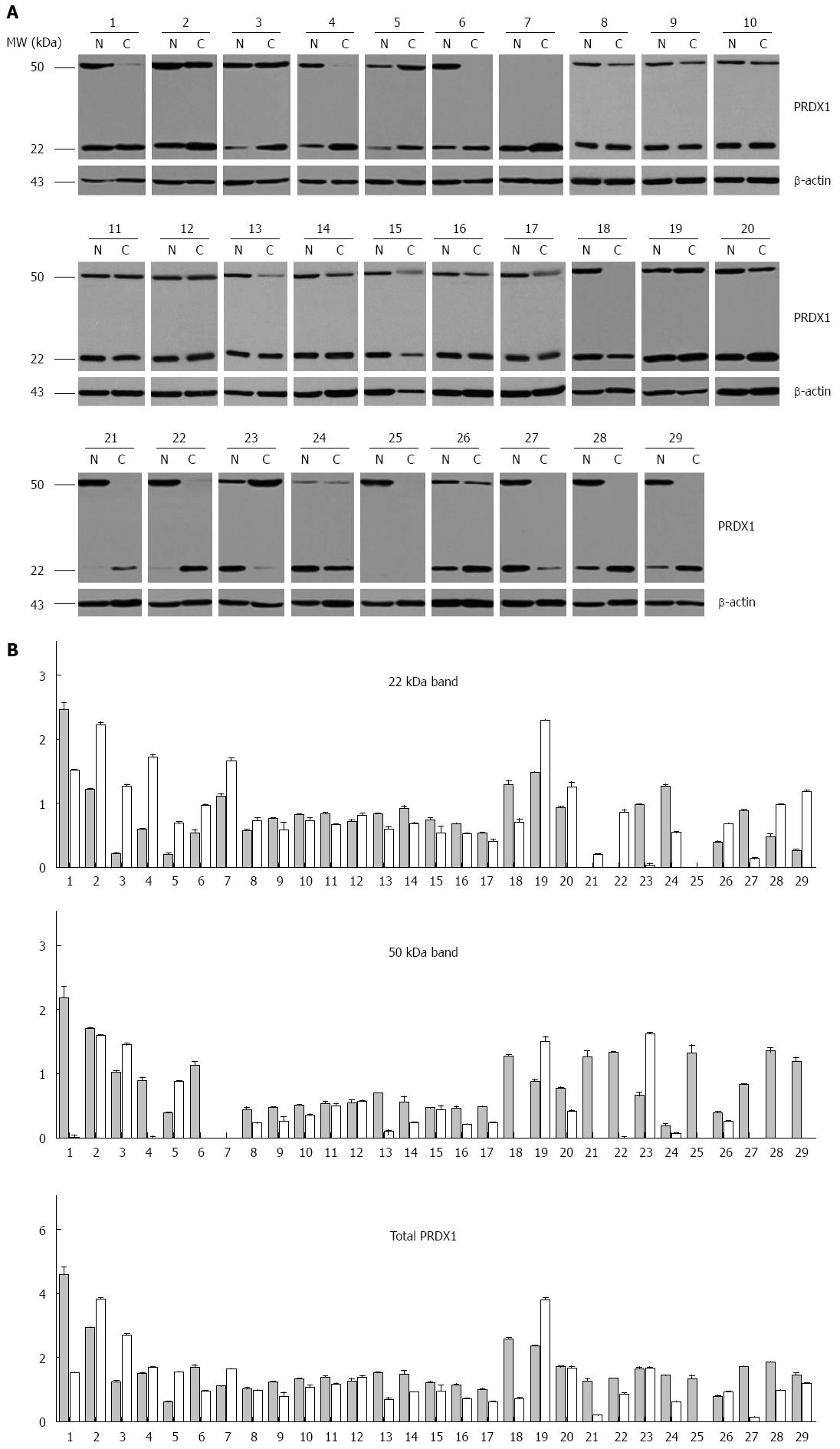
Figure 3 Expression of peroxiredoxin 1 in human liver cancer specimens.
A: Western blot analysis of tumor (C) and matching adjacent non-tumor liver tissues (N) of 29 patients. β-actin protein levels are shown as a loading control. The patients were coded from 1 to 29; B: Densitometric analysis of 29 hepatocellular carcinoma cases. The black and gray bars represent the relative band intensity of peroxiredoxin 1 (PRDX1) in non-tumor or tumor tissues, showing the ratio between 22 kDa, 50 kDa or total PRDX1 and β-actin. Each data point represents the mean ± SD derived from three independent experiments.
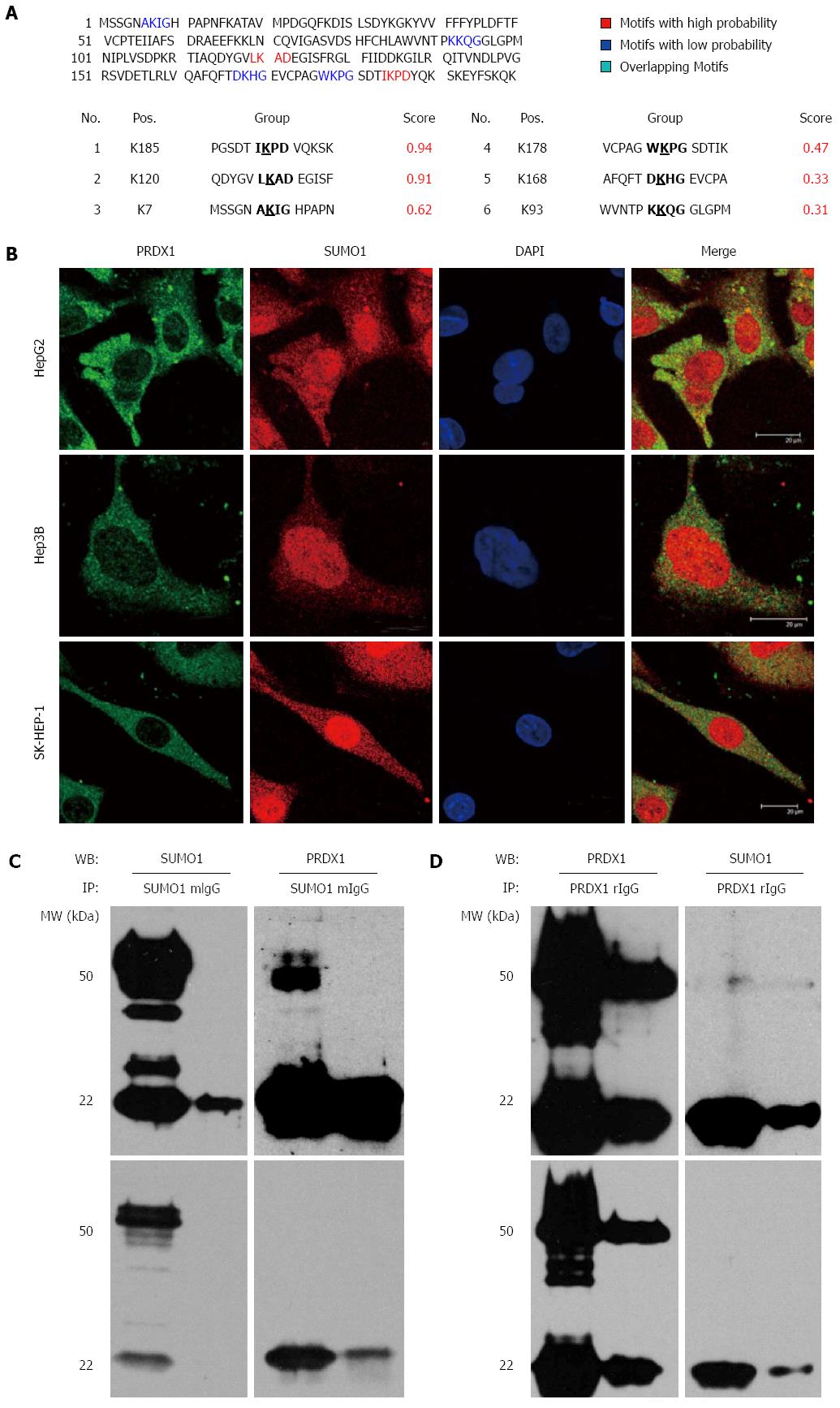
Figure 4 Peroxiredoxin 1 might be sumoylated in liver cancer cells.
A: The bioinformatic prediction of PRDX1 using SUMOplot tool; B: Immunofluorescence staining visualized under a confocal microscope illustrating the co-localization of PRDX1 and SUMO1 proteins in the cytoplasm of three liver cancer cells. C, D: Co-immunoprecipitation of PRDX1 with SUMO1 in HepG2 cell extract; C: Lysates were subjected to immunoprecipitation (IP) with anti-SUMO1 antibody, followed by Western blotting (WB) with anti-PRDX1 and anti-SUMO1 to detect sumoylated PRDX1; D: Lysates were subjected to IP with anti-PRDX1 antibody, followed by WB with anti-SUMO1 and anti-PRDX1 to detect sumoylated PRDX1. The upper and lower panels are the results of dark and light exposure by Western blotting.
- Citation: Sun YL, Cai JQ, Liu F, Bi XY, Zhou LP, Zhao XH. Aberrant expression of peroxiredoxin 1 and its clinical implications in liver cancer. World J Gastroenterol 2015; 21(38): 10840-10852
- URL: https://www.wjgnet.com/1007-9327/full/v21/i38/10840.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i38.10840