Copyright
©The Author(s) 2015.
World J Gastroenterol. Jan 14, 2015; 21(2): 653-660
Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.653
Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.653
Figure 1 Study design.
ADV: Adefovir dipivoxil; HBV: Hepatitis B virus; LAM: Lamivudine; CHB: Chronic hepatitis B.
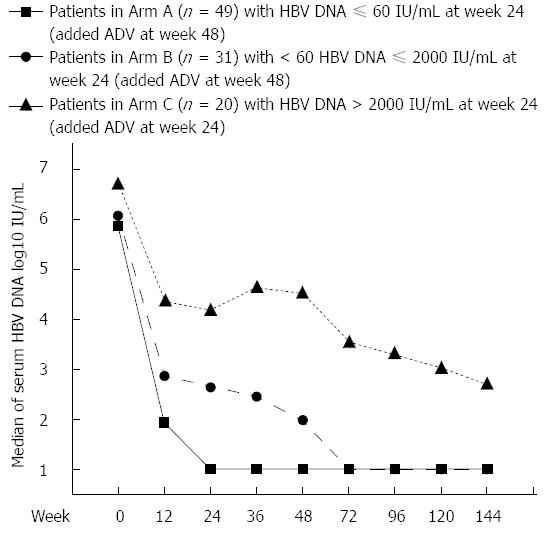
Figure 2 Pattern of hepatitis B virus DNA decline in three arms during 144 wk nucleos(t)ide analogue treatment.
ADV: Adefovir dipivoxil; HBV: Hepatitis B virus.
Figure 3 Undetectable rates of serum hepatitis B virus DNA at different time points in three arms.
ADV: Adefovir dipivoxil; HBV: Hepatitis B virus.
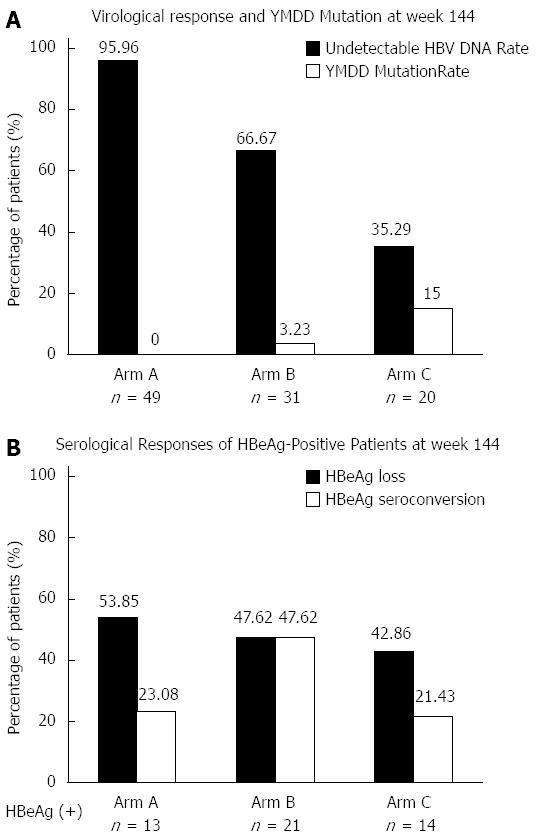
Figure 4 Undetectable rates of serum hepatitis B virus DNA levels, YMDD mutation rates, hepatitis B e antigen loss and hepatitis B e antigen seroconversion rates at week 144 in three arms.
A: Virological response and YMDD Mutation at week 144; B: Serological responses of hepatitis B e antigen (HBeAg)-positive patients at week 144.
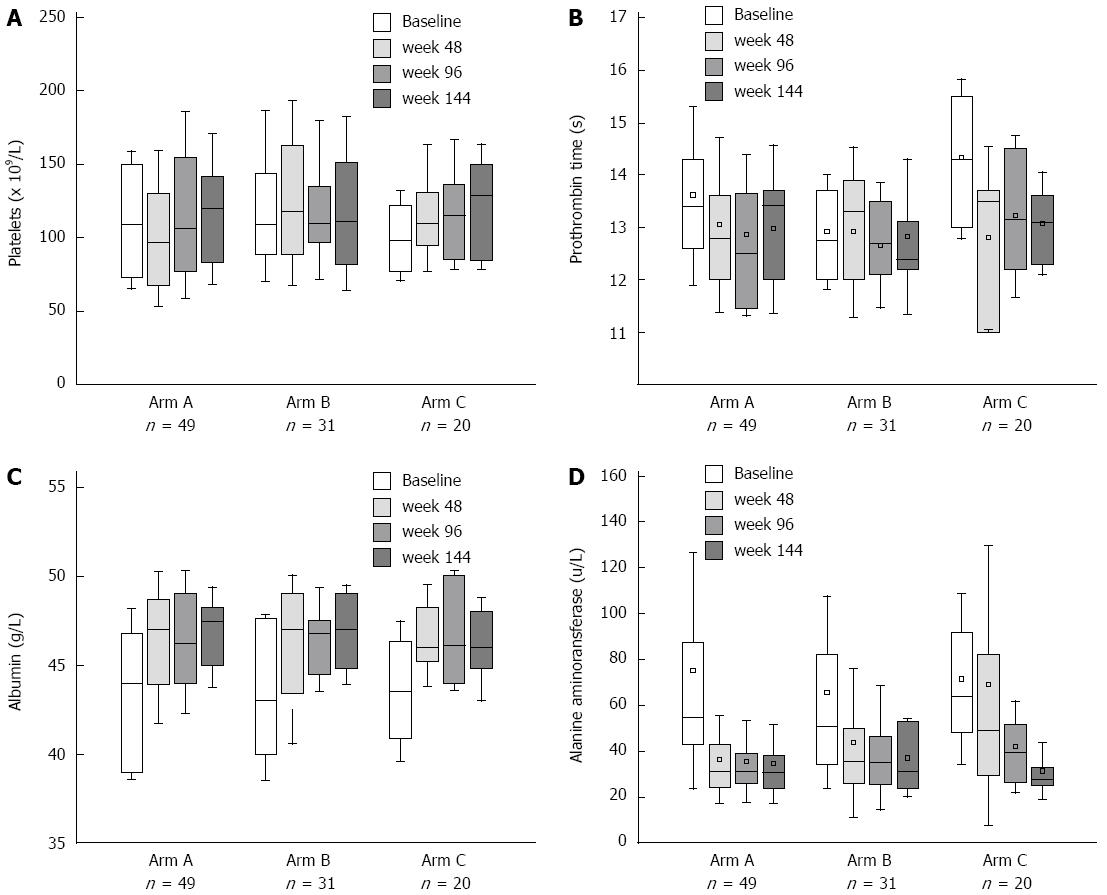
Figure 5 Changes after nucleos(t)ide analogue treatment in three arms.
A: Platelet counts; B: Prothrombin time; C: Albumin; D: Alanine aminotransferase.
- Citation: Gu EL, Yu YQ, Wang JL, Ji YY, Ma XY, Xie Q, Pan HY, Wu SM, Li J, Chen CW, Xu XW, Wang YE, Yao GB, Wang H, Zhang WH. Response-guided treatment of cirrhotic chronic hepatitis B patients: Multicenter prospective study. World J Gastroenterol 2015; 21(2): 653-660
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.653