Copyright
©The Author(s) 2015.
World J Gastroenterol. Jan 14, 2015; 21(2): 584-592
Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.584
Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.584
Figure 1 Flow of enrollment of study participants.
HBsAg: Hepatitis B surface antigen; LT: Liver transplantation; im: Intramuscular; HBIG: Hepatitis B immunoglobulin; LAM: Lamivudine; ETV: Entecavir; ADV: Adefovir dipivoxil.
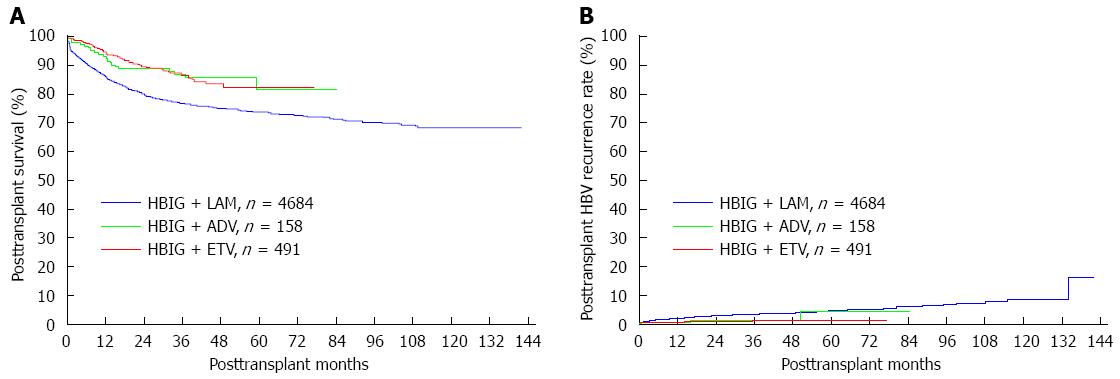
Figure 2 Cumulative posttransplant survival and hepatitis B virus recurrence rates for each group.
A: Cumulative posttransplant survival; B: Cumulative posttransplant hepatitis B virus (HBV) recurrence. HBIG: Hepatitis B immunoglobulin; LAM: Lamivudine; ETV: Entecavir; ADV: Adefovir dipivoxil.
- Citation: Shen S, Jiang L, Xiao GQ, Yan LN, Yang JY, Wen TF, Li B, Wang WT, Xu MQ, Wei YG. Prophylaxis against hepatitis B virus recurrence after liver transplantation: A registry study. World J Gastroenterol 2015; 21(2): 584-592
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/584.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.584