Copyright
©The Author(s) 2015.
World J Gastroenterol. Apr 21, 2015; 21(15): 4644-4651
Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4644
Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4644
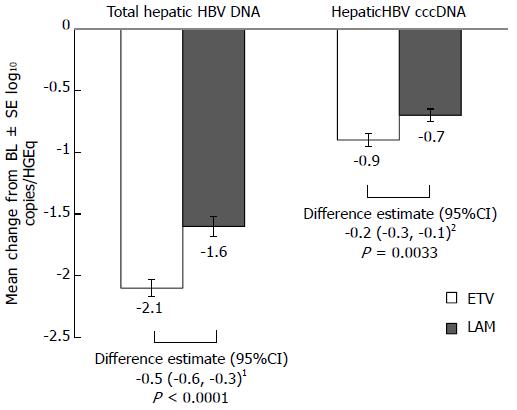
Figure 1 Change from baseline at week 48 in total hepatic hepatitis B virus DNA and hepatitis B virus covalently closed-circular DNA.
1Adjusted for baseline total hepatic HBV DNA level; 2Adjusted for baseline hepatic cccDNA level. BL: Baseline; CI: Confidence interval; HGEq: Human genome equivalent; SE: Standard error; CccDNA: Covalently closed-circular DNA; ETV: Entecavir; LAM: Lamivudin.
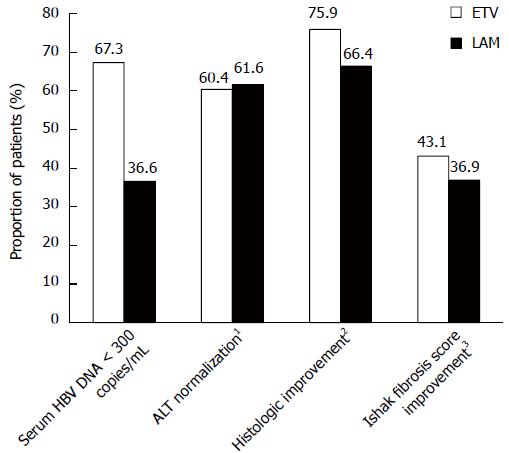
Figure 2 Virologic, biochemical, and histologic efficacy at week 48 among treated patients with evaluable hepatic hepatitis B virus DNA pairs.
1ALT < 1.25 × upper limit of normal; 2≥ 2-point decrease in Knodell necroinflammatory score with no worsening (≥ 1-point increase from baseline) of Knodell fibrosis score; 3≥ 1-point decrease in Ishak fibrosis score from baseline.
- Citation: Bowden S, Locarnini S, Chang TT, Chao YC, Han KH, Gish RG, de Man RA, Yu M, Llamoso C, Tang H. Covalently closed-circular hepatitis B virus DNA reduction with entecavir or lamivudine. World J Gastroenterol 2015; 21(15): 4644-4651
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4644