Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Dec 28, 2014; 20(48): 18189-18198
Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18189
Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18189
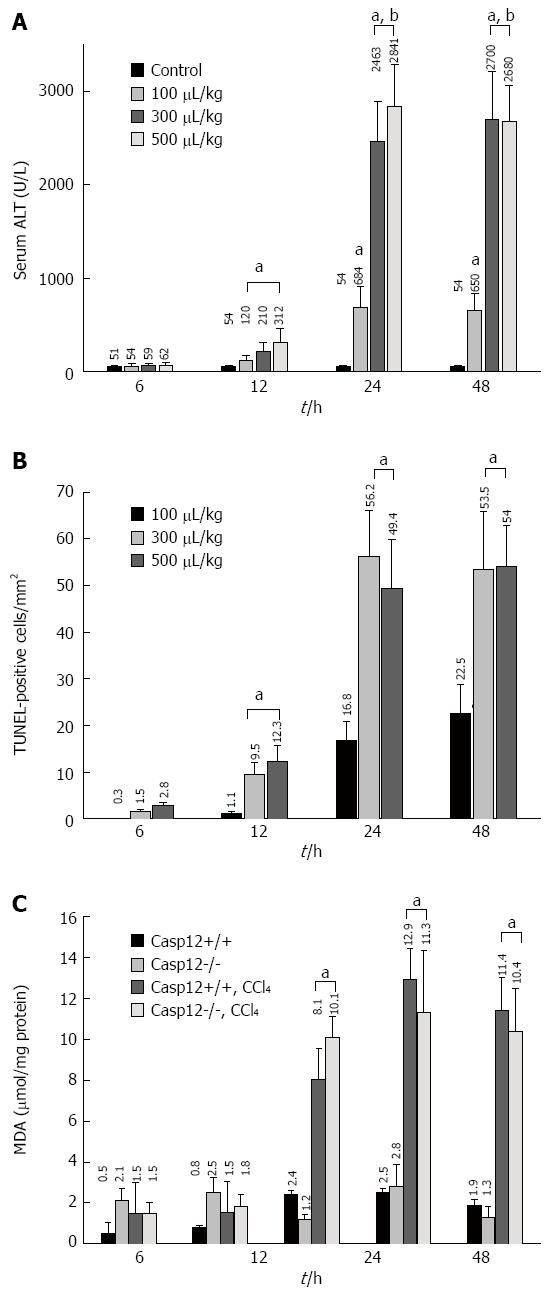
Figure 1 Dose- and time-dependent liver damage, apoptosis and reactive oxygen species generation.
A: Carbon tetrachloride (CCl4)-induced hepatic injury as measured by alanine aminotransferase (ALT) levels. Values are mean ± SE, n = 5, aP < 0.05 vs control, cP < 0.05 vs 12 h at the same dose; B: Hepatic apoptosis was examined by terminal transferase-mediated dUTP nick-end labeling (TUNEL) staining. For each sample, five randomly selected fields at 200× magnification were evaluated. Values are mean ± SE, n = 5; eP < 0.05 vs 100 μL/kg body weight group at the same time point; C: Generation of reactive oxygen species as measured by malondialdehyde (MDA) in both caspase-12+/+ and -12-/- mouse livers 12, 24 and 48 h following CCl4 treatment (300 μL/kg body weight). Values are mean ± SE, n = 8; gP < 0.05 vs controls.
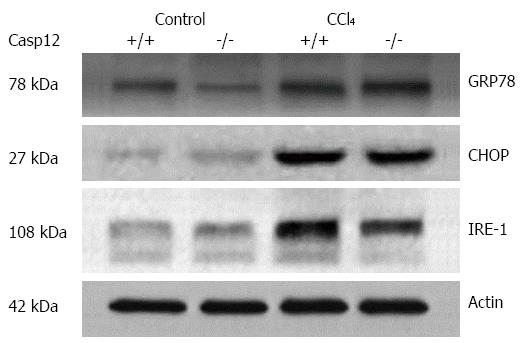
Figure 2 Carbon tetrachloride induced endoplasmic reticulum stress in the liver.
The representative Western blots show that there was an increase in endoplasmic reticulum stress response in CCl4-treated mice. Glucose-regulated protein 78 (GRP78), CCAAT/-enhancer-binding protein homologous protein (CHOP) and serine/threonine-protein kinase/endoribonuclease (IRE-1) were upregulated in the livers of both caspase-12+/+ and -12-/- mice treated with CCl4.
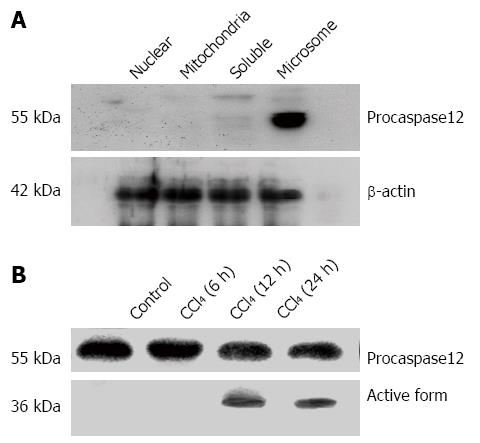
Figure 3 Localization of procaspase-12 in the subcellular components of liver cells and the activation of caspase-12 by carbon tetrachloride.
A: Procaspase-12 was detected only in the microsome fraction but not in the nuclear, mitochondria and soluble fractions; B: Activation of caspase-12 was detected in the liver of caspase-12+/+ mice at 12 and 24 h after CCl4 treatment. Western blot was performed using an antibody recognizing both pro- and active forms of caspase-12. Procaspase-12 (55 kDa) was cleaved to the active form (36 kDa). The representative blot was from the results of three experiments.
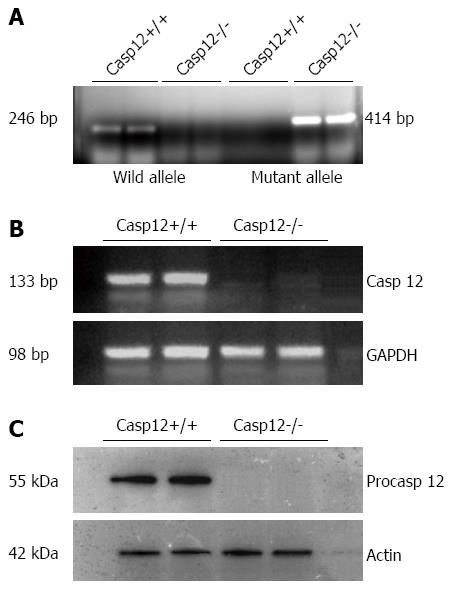
Figure 4 Genotyping and confirmation of the lack of caspase-12 in the caspase-12-/- mice.
A: Genotyping result indicates the absence of wild-type allele and presence of mutant allele in the caspase-12-/- mice; B: Reverse transcription polymerase chain reaction confirmed the absence of caspase-12 mRNA in caspase-12-/-; C: Western blot showing the absence of caspase-12 protein in caspase-12-/- mice.
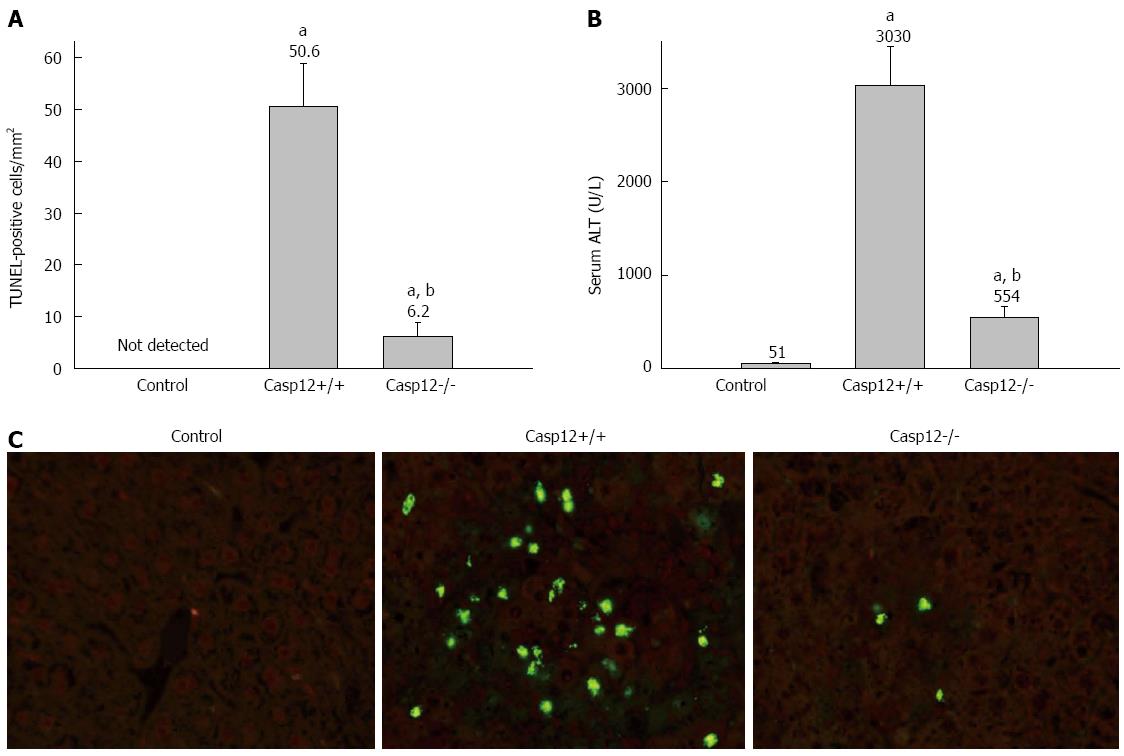
Figure 5 Attenuated carbon tetrachloride-induced hepatic apoptosis and liver injury in caspase-12-/- mice.
A: Terminal transferase-mediated dUTP nick-end labeling (TUNEL) staining to evaluate carbon tetrachloride (CCl4)-induced hepatocyte apoptosis in caspase-12-/- mice. Five randomly selected fields at 200× magnification were evaluated for each tissue section. The numbers of apoptotic cells were counted as TUNEL-positive cells/mm2. Values are mean ± SE, n = 8; aP < 0.05 vs controls, cP < 0.05 vs caspase-12+/+; B: CCl4-induced liver injury as measured by serum alanine aminotransferase (ALT) levels. Values are mean ± SE, n = 8; eP < 0.05 vs controls, gP < 0.05 vs caspase-12+/+ mice; C: Representative sections of TUNEL staining under fluorescence microscope (200 ×). TUNEL-positive cells were identified by the nuclear fluorescence staining.
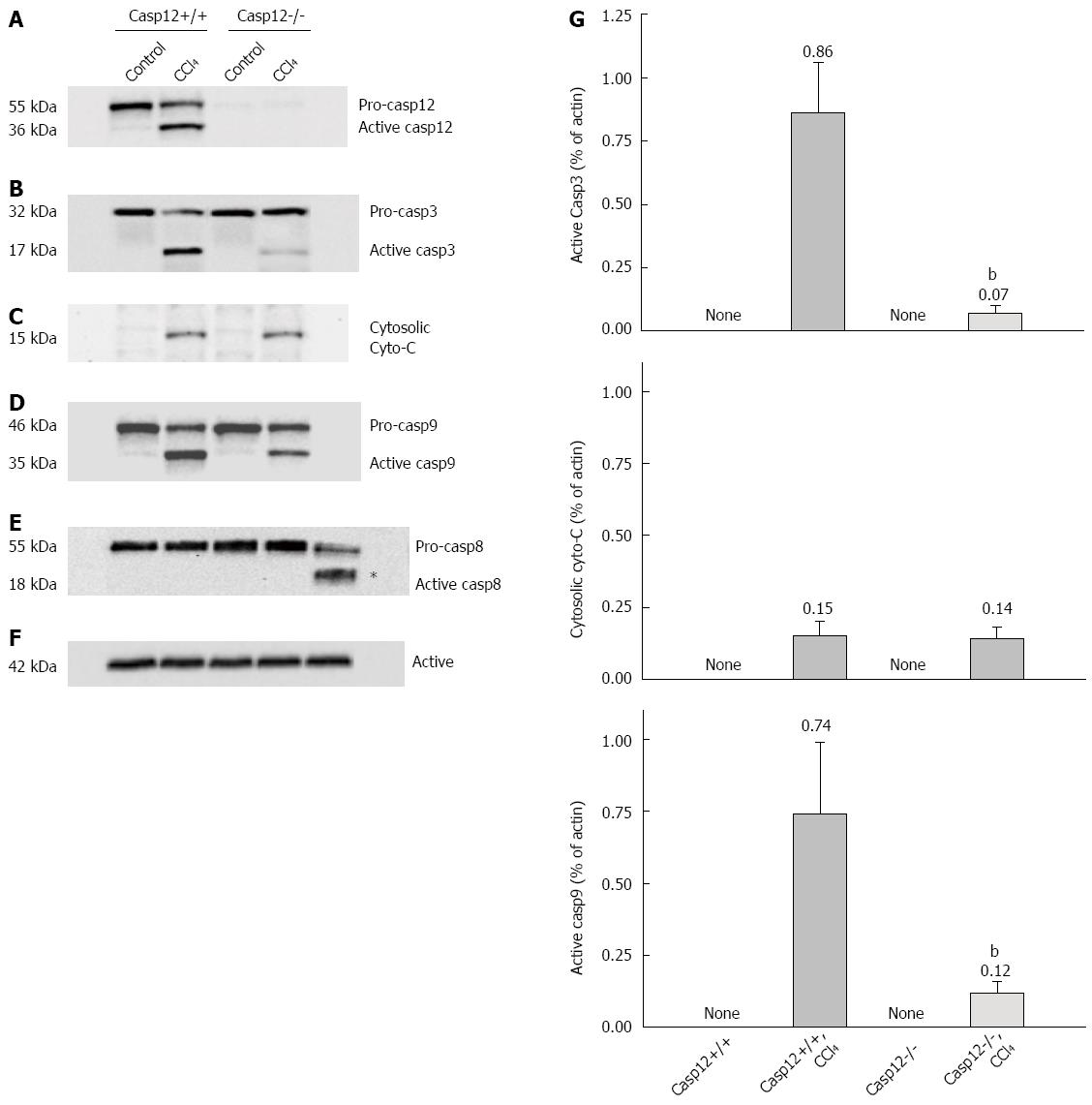
Figure 6 Activation of caspases and cytochrome C release in mice treated with carbon tetrachloride.
Western blot analysis of A: Caspase-12; B: Caspase-3; C: Cytochrome C release from mitochondria; D: Casapse-9; E: Caspase-8; F: Actin as control in CCl4-treated and control caspase-12+/+ and caspase-12-/- mice; G: Densitometric analyses of cytosolic cytochrome C and cleaved caspase-3 and -9 were performed from four independent Western blot experiments. The values were normalized to actin for each individual sample. Values are mean ± SE; aP < 0.05 vs CCl4-treated caspase-12+/+.
- Citation: Liu H, Wang Z, Nowicki MJ. Caspase-12 mediates carbon tetrachloride-induced hepatocyte apoptosis in mice. World J Gastroenterol 2014; 20(48): 18189-18198
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18189