Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 14, 2014; 20(42): 15467-15475
Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15467
Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15467
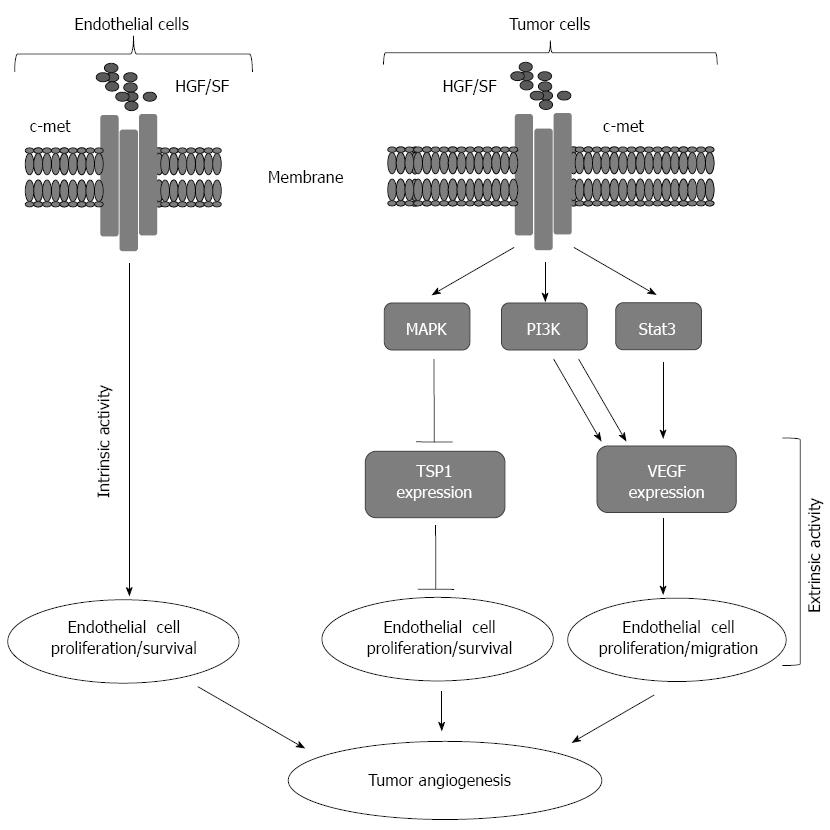
Figure 1 Representative model for tumor angiogenesis induced by hepatocytes growth factor/Scater factor-Met signaling.
Intrinsically hepatocytes growth factor/Scater factor (HGF/SF) activates Met receptor on the surface of host endothelial cells leading to cell proliferation and migration. Extrinsically, HGF/SF-Met signaling turns on the angiogenic switch by simultaneous upregulation of pro-angiogenic vascular endothelial growth factors (VEGF) expression and down regulation of thrombospondin 1 (TSP-1) expression from the tumor cells.
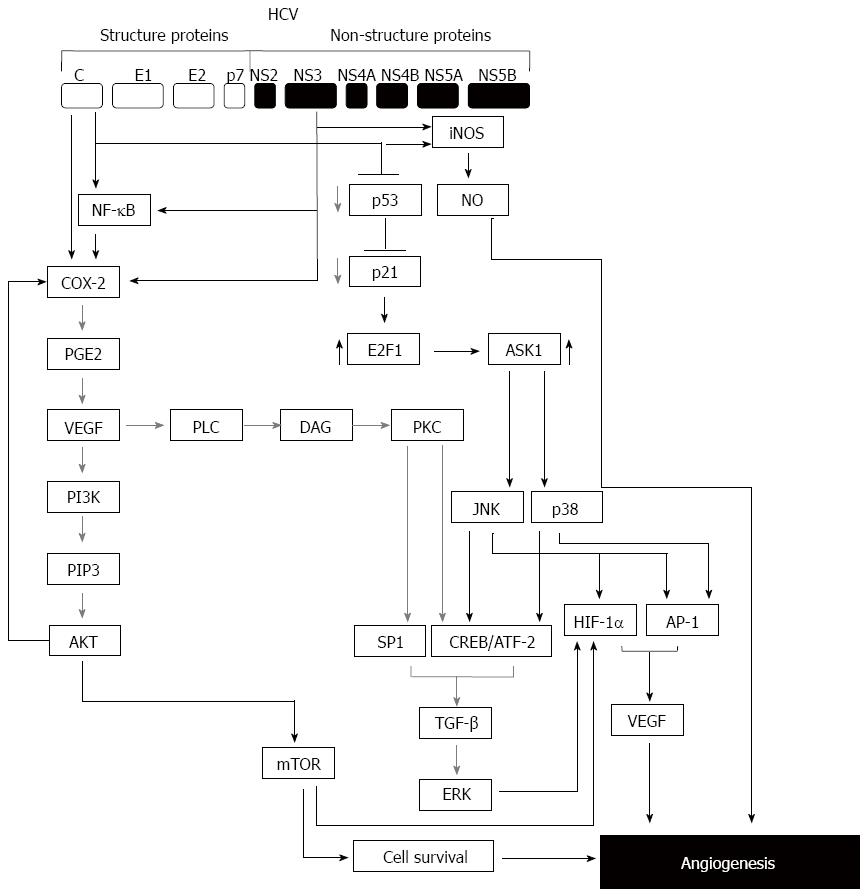
Figure 2 Model for hepatitis C virus-mediated hepatic angiogenesis.
During the infection with hepatitis C virus (HCV), normal angiogenesis process can be malignant through the deregulation of genes involved in the angiogenic pathway by viral proteins such as core and non-structural protein NS3. HCV infection can enhance angiogenic process via multiple pathways. One of these pathways is initiated by HCV core or NS3 via NF-κB and, cyclooxygenase (COX-2) leading to the activation of vascular endothelial growth factor (VEGF)/PI3K/AKT/mTOR axis. The other pathway is initiated by core and NS3-induced iNOS/NO axis leading to angiogenesis. Further pathway is initiated by HCV-induced suppression of p53-p21 axis leading to the induction of E2F1 that subsequently mediates the activation of ASK1-JNK/p38 that results in the induction of TGF-β leading to the activation of extracellular regulated kinase (ERK) pathway. ERK pathway together with c-Jun-N-terminal kinase (JNK), p38 will be able to trigger the expression of VEGF and subsequently to the promotion of hepatic angiogenesis.
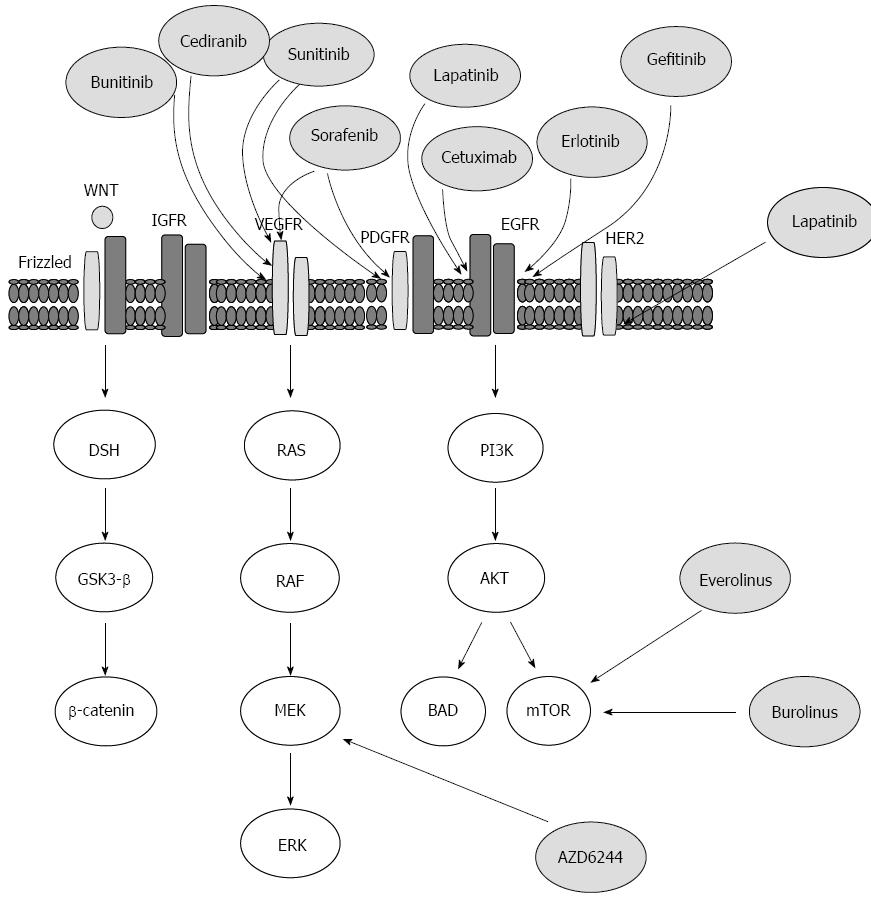
Figure 3 Outline of the targeted therapies, which are currently available or under development for the treatment of hepatocellular carcinoma, and the molecular targets on which they are believed to act upon.
AKT: A protein kinase family of genes involved in regulation of cell survival, Bcl-2-associated agonist of cell death promoter (BAD), Bcl-2-associated death promoter; Disheveled (DSH) protein, downstream effector Disheveled; EGF: Epidermal growth factor; EGFR: EGF receptor; ERK: Extracellular signal-regulated kinase; Frizzled: A family of G-protein coupled receptor proteins that serve as receptors in the WNT/β-catenin signaling pathway; once activated: Frizzled leads to activation of Disheveled in the cytoplasm; GSK-3β: Glycogen synthase kinase 3β; HER2/neu: Human epidermal growth factor receptor 2, a cell membrane surface-bound receptor tyrosine kinase that is involved in the signal transduction pathways leading to cell growth and differentiation; MEK: Kinases that phosphorylate mitogen activated protein (MAP) kinase (MAPK); mTOR: Mammalian target of rapamycin; PDGFR: Platelet-derived growth factor receptor; PI3K: Phosphatidylinositol-3-kinase; PTEN: Phosphatase and tensin homolog, regulates cell-survival pathway; RAF: A MAP kinase kinase kinase (MAP3K) that functions in the MAPK/ERK signal transduction pathway; a serine/threonine-specific kinase; RAS: Prototypical member of the RAS superfamily of proteins; activation of RAS signaling causes cell growth, differentiation and survival; the dysregulation of RAS signaling can lead to oncogenesis and cancer.
- Citation: Hassan M, Selimovic D, El-Khattouti A, Soell M, Ghozlan H, Haikel Y, Abdelkader O, Megahed M. Hepatitis C virus-mediated angiogenesis: Molecular mechanisms and therapeutic strategies. World J Gastroenterol 2014; 20(42): 15467-15475
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15467