Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 7, 2014; 20(41): 15018-15027
Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15018
Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15018
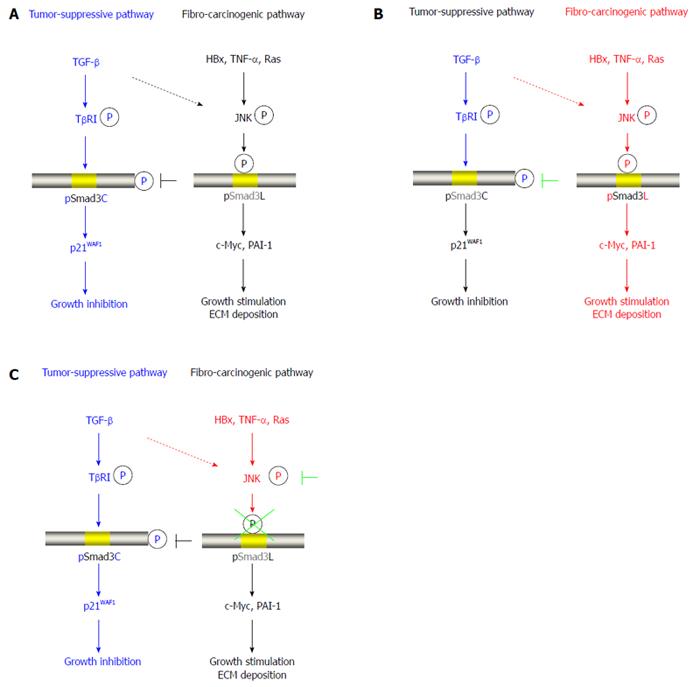
Figure 1 Reversibility of phospho-Smad3 signaling between tumor suppression and fibro-carcinogenesis.
A: Additional treatment of transforming growth factor-β (TGF-β) activates TGF-β receptor type I (TβRI), further leading to direct phosphorylation of Smad3C, which inhibits normally hepatocytic growth by upregulating p21WAF1 transcription; B: Mitogens drastically alter phospho-Smad3 signaling via the c-Jun N-terminal kinase (JNK) pathway, increasing nuclear fibro-carcinogenic pSmad3L activity while shutting down TGF-β-dependent cytostatic pSmad3C. Although the TGF-β signal weakly phosphorylates Smad3L in normal hepatocytes (dotted line), hepatitis viral components, including HBx, pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), and somatic mutations such as Ras, additively transmit a fibro-carcinogenic signal through the JNK-dependent pSmad3L pathway to participate in hepatocytic growth and ECM deposition, possibly by stimulating transcription of c-Myc and PAI-1 genes. Linker phosphorylation of Smad3 indirectly prevents COOH-tail phosphorylation, pSmad3C-mediated p21WAF1 transcription and cytostatic function; C: Either various JNK inhibitors or a Smad3 mutation causing lack of JNK phosphorylation sites in the linker region can eliminate fibro-carcinogenic pSmad3L signaling, restoring or maintaining the tumor-suppressive pSmad3C signaling characteristic of mature hepatocytes.
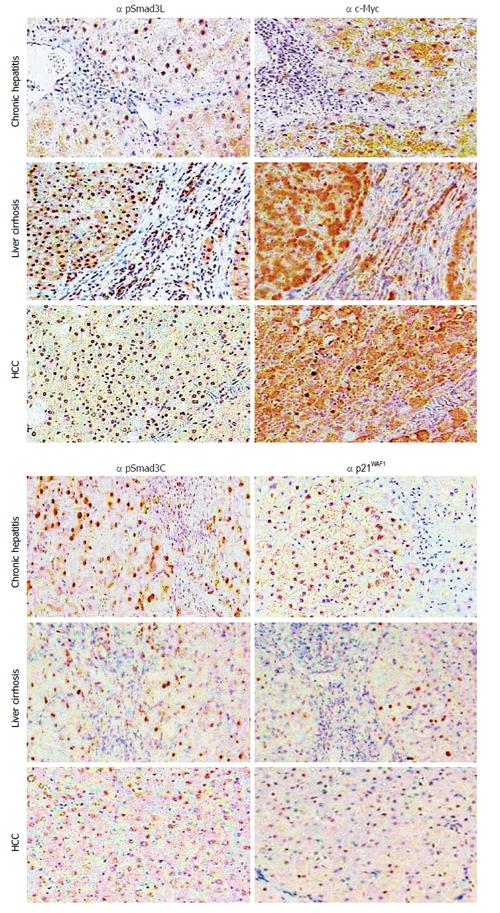
Figure 2 Hepatic fibro-carcinogenesis: reciprocal change in linker phosphorylated and COOH-terminally phosphorylated Smad3 pathways.
The linker phosphorylated Smad3 (pSmad3L)/c-Myc pathway shows increasing prominence in hepatocytes, as hepatitis C virus (HCV)-infected liver progresses from chronic hepatitis, through cirrhosis to hepatocellular carcinoma (HCC). In contrast to the intense staining for pSmad3L and c-Myc, the COOH-terminally phosphorylated Smad3 (pSmad3C)/p21WAF1 pathway staining decreases in hepatocytes as liver disease progresses toward HCC.
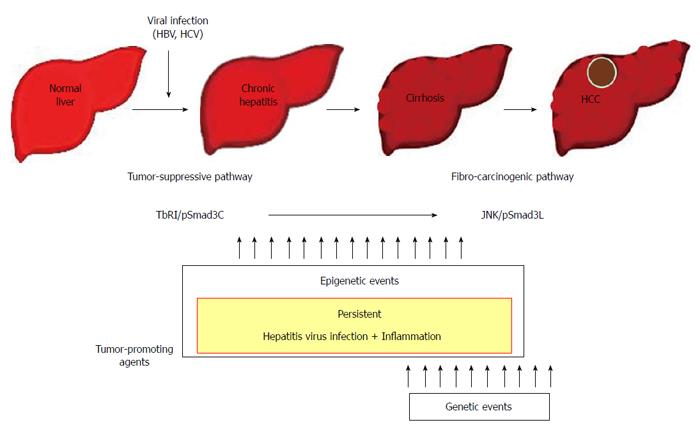
Figure 3 Contribution to fibro-carcinogenesis by tumor-promoting agents.
Together, persistent hepatitis virus infection and chronic inflammation shift hepatocytic phospho-Smad3 signaling from the tumor-suppressive transforming growth factor-β (TGF-β) receptor type I (TβRI)/COOH-terminally phosphorylated Smad3 (pSmad3C) mode to the fibro-carcinogenic c-Jun N-terminal kinase (JNK)/linker phosphorylated Smad3 (pSmad3L) mode characteristic of median forebrain bundle, accelerating liver fibrosis while increasing risk of hepatocellular carcinoma (HCC). In the liver, persistent hepatitis virus infection and chronic inflammation contribute to fibro-carcinogenesis. In proportion to the severity of fibrosis, mitogenic genetic or epigenetic alterations can drive fibro-carcinogenesis via the pSmad3L pathway. Escaping the cytostatic action of pSmad3C is a critical step for progression to full malignancy in cancers, which must overcome multiple fail-safe genetic controls. HBV: Hepatitis B virus; HCV: Hepatitis C virus.
- Citation: Murata M, Yoshida K, Yamaguchi T, Matsuzaki K. Linker phosphorylation of Smad3 promotes fibro-carcinogenesis in chronic viral hepatitis of hepatocellular carcinoma. World J Gastroenterol 2014; 20(41): 15018-15027
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15018.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15018