Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Sep 14, 2014; 20(34): 12118-12131
Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12118
Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12118
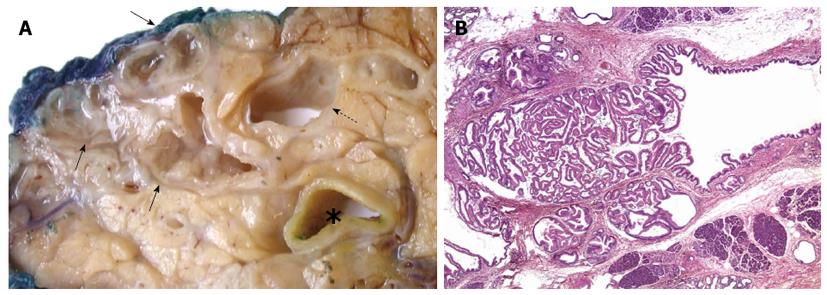
Figure 1 Intraductal papillary mucinous neoplasia.
A: The main pancreatic duct (dotted arrow) and a cluster of branch ducts (arrows) are dilated, and particularly the latter contain mucus and a papillary proliferation on the duct walls (asterisk, common bile duct); B: Partial involvement of a pancreatic branch duct by the papillary proliferation of neoplastic epithelium characteristic of intraductal papillary mucinous neoplasia (HE, 20 × magnification).
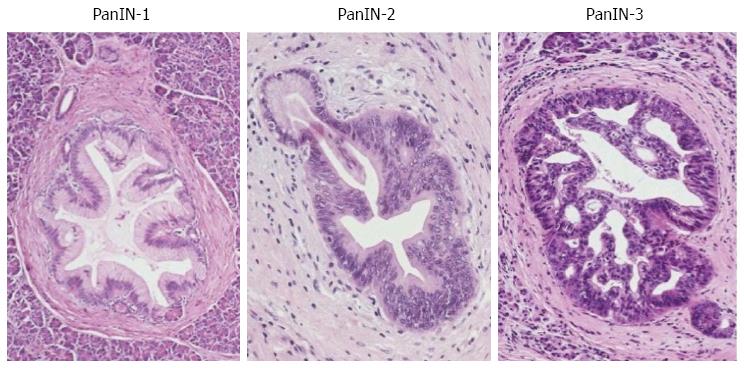
Figure 2 Pancreatic intraepithelial neoplasia.
Small pancreatic branch ducts are involved by a low-papillary proliferation of neoplastic columnar epithelium showing mild, moderate and severe dysplasia corresponding to pancreatic intraepithelial neoplasia (PanIN)-1, PanIN-2 and PanIN-3.
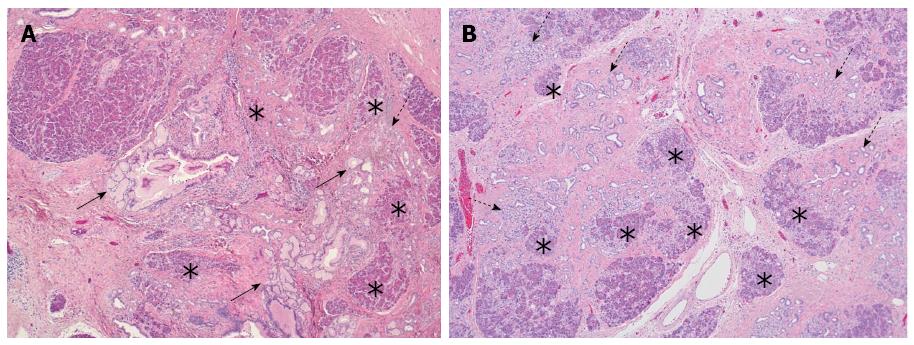
Figure 3 Lobulocentric atrophy.
A: Lobules of acinar parenchyma are atrophic (asterisk) and partially replaced by tubular structures (so-called acinar to ductal metaplasia; dotted arrow) and fibrosis. Note the foci of PanIN-1 in the centre of the changes (arrows); B: Lobulocentric atrophy of neighbouring lobules (asterisk) results in a large area of fibrosis with tubular structures (dotted arrows) but without PanIN-lesion.
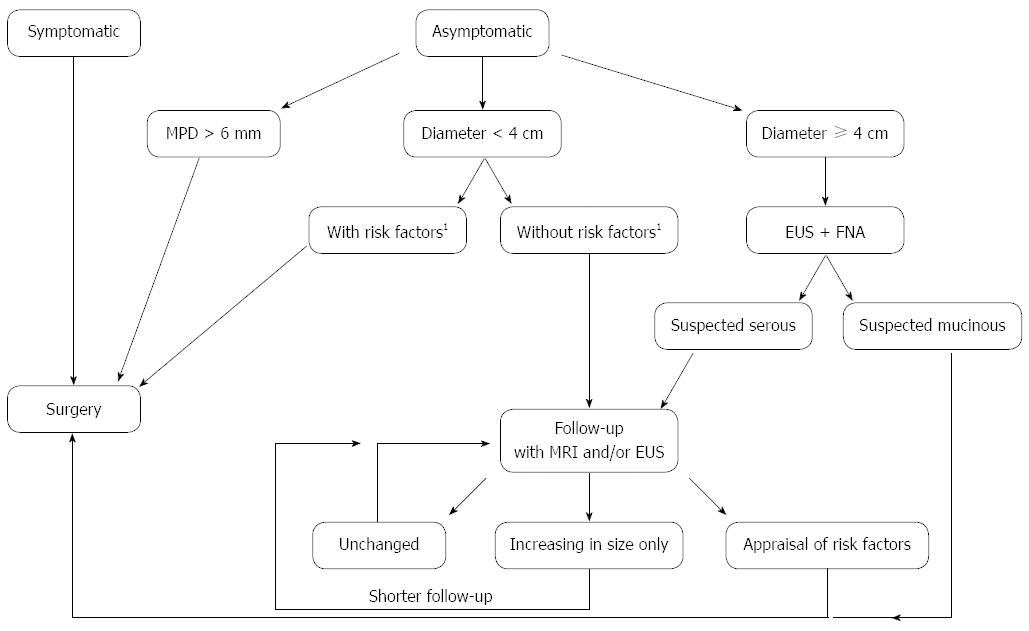
Figure 4 Algorithm that combines the current European and international guidelines for intraductal papillary mucinous neoplasia of the pancreas.
FNA: Fine needle aspiration; MPD: Main pancreatic duct; EUS: Endoscopic ultrasound. Risk factors1: Mural nodules, increased serum levels of Ca 19.9, rapid increase in size.
- Citation: Chiaro MD, Segersvärd R, Lohr M, Verbeke C. Early detection and prevention of pancreatic cancer: Is it really possible today? World J Gastroenterol 2014; 20(34): 12118-12131
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12118