Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Sep 7, 2014; 20(33): 11467-11485
Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11467
Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11467
Figure 1 David Y Graham, MD, Professor, Department of Medicine, Michael E.
DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, 2002 Holcombe Blvd, Houston, TX 77030, United States.
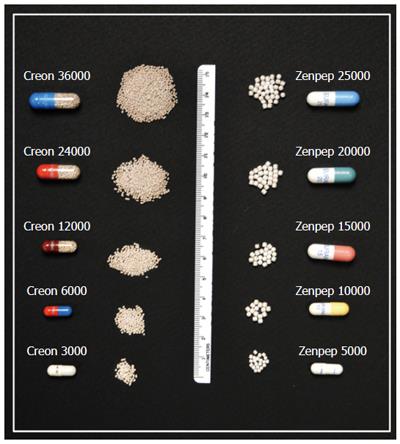
Figure 2 Pancreatic enzyme capsule size and contents increase as the pancreatic enzyme preparation dosage increases, suggesting that dose/unit increases are achieved by packaging the same basic pancreatic enzyme formulation into a larger capsule and/or larger beads.
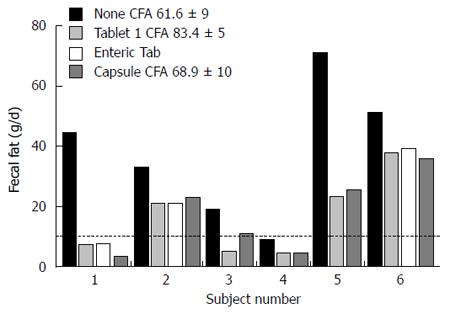
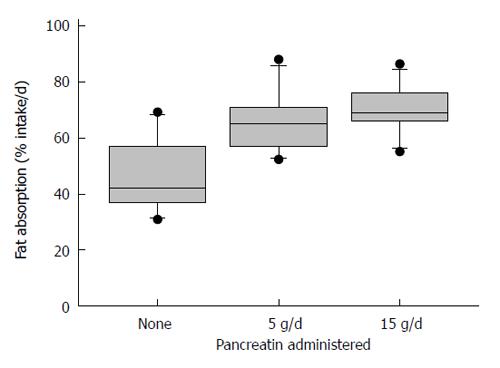
Figure 3 Results of different pancreatic enzyme preparations, tablets, enteric-coated tablets, and capsule in adults with exocrine pancreatic insufficiency.
Approximately 30000 USP units of lipase were given with meals. Steatorrhea was corrected in those with low acid secretion. From[28] with permission.
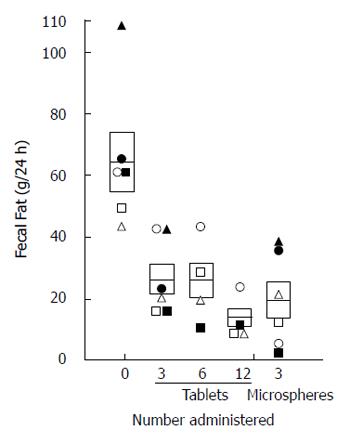
Figure 4 Effect of increasing the enzyme dosage on fecal fat excretion on a 100 gram fat diet.
Enzymes were given 3 times per day with meals providing approximately 30000, 60000, or 120000 USP lipase units with each meal or as 18000 USP lipase units as enteric coated microspheres (i.e., 3 tablets, 6 tablets or 12 tablets and 3 microsphere capsules with each meal). Each rectangle encloses the mean ± the standard deviation of the mean. The normal fecal fat is < 6 g/24 h. From[29] with permission.
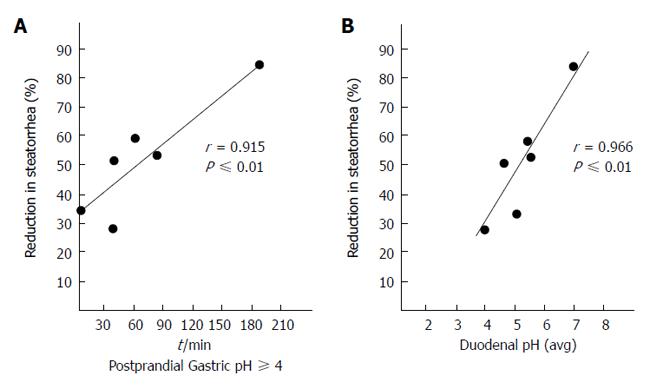
Figure 5 Correlation between percentage reduction in steatorrhea based on the median obtained with 30000 USP units of lipase given in tablets or capsules compared with the time of the postprandial gastric pH was > 4 (A) and in those same subjects compared with the mean post prandial duodenal pH (B).
From[28] with permission.
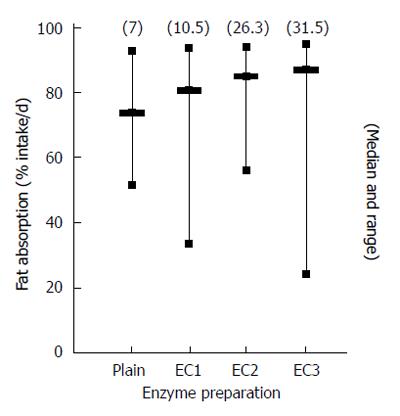
Figure 6 Results of a study comparing the response of enteric coated pancreatic enzyme in a cystic fibrosis patient population.
Different doses of enteric coated pancreatic enzymes were taken four times daily immediately before meals and the corresponding average % fat absorption/d[11].
Figure 7 Randomized cross-over study in patients with cystic fibrosis and pancreatic insufficiency that compared plain uncoated enzymes (Pancrex V Forte n = 14) and 3 different enteric coated preparations [EC1:Pancreatin Merk, EC2: Creon and EC3: Pancrease (n = 19)] using the same lipase dosage.
The median and range are shown of fecal fat absorption. With the numbers above the columns ( ) indicating the percent of patients with > 90% fat absorption. None reliably resulted in normalization of fat malabsorption[42].
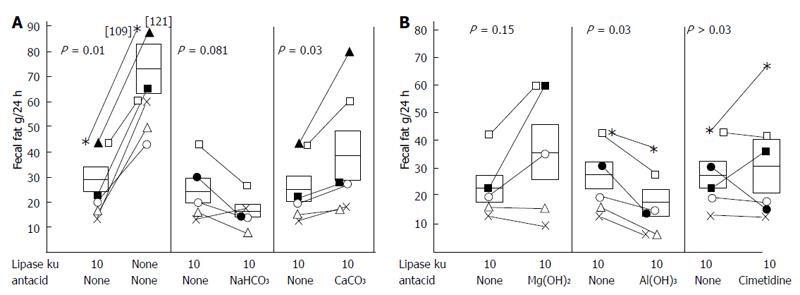
Figure 8 Effect of antacids and enzymes on the effectiveness of 30000 USP units of lipase per meal for the treatment of pancreatic steatorrhea.
Each symbol represents a different patient. Sodium bicarbonate, magnesium aluminum hydroxide, aluminum hydroxide, or calcium carbonate were administered at the beginning and the termination of each meal. Cimetidine was given 30 min prior to the meal. From[58] with permission.
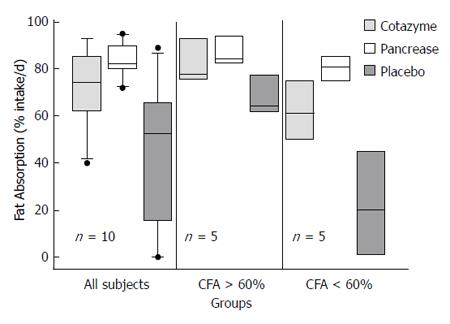
Figure 9 Randomized cross-over comparison of similar amounts of lipase administered as unprotected capsule (Cotazyme®) or enteric coated microspheres (Pancrease®) in cystic fibrosis patients with pancreatic insufficiency.
Although the enteric coated preparation was better in those with the greatest degree of malabsorption, neither resulted in resolution of steatorrhea[61].
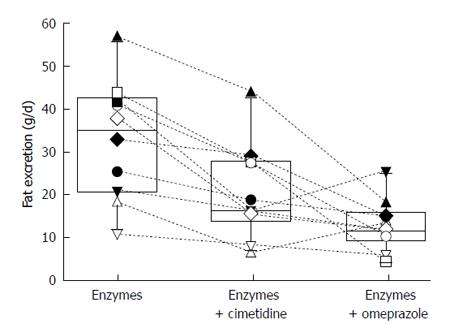
Figure 10 Box plot showing median and 25% and 75% and range for a randomized cross-over study comparing the effect of 1200 mg cimetidine or 60 mg of omeprazole on the effectiveness of pancreatic enzymes.
Six tablets of unprotected enzymes (Cotazyme Forte® 36000 FIP units/meal) given 1/2 before meal and 1/2 during the meal). Both antisecretory agents improved outcome but neither reliably resolved steatorrhea. Data from[72].
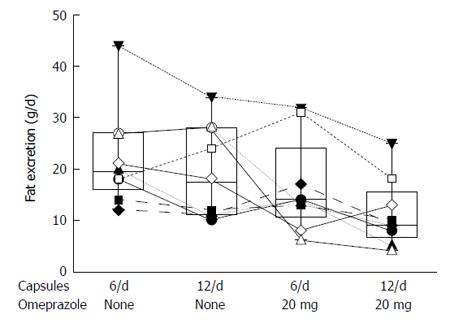
Figure 11 Box plot showing median and 25% to 75% range for a randomized cross-over study comparing the effect of doubling the dose of pancreatic enzyme microspheres (Pancrease®) and the effect of omeprazole in patients with cystic fibrosis and pancreatic insufficiency.
Enzymes were taken 1/2 just before and 1/2 after meals. Omeprazole 20 min before breakfast[67].
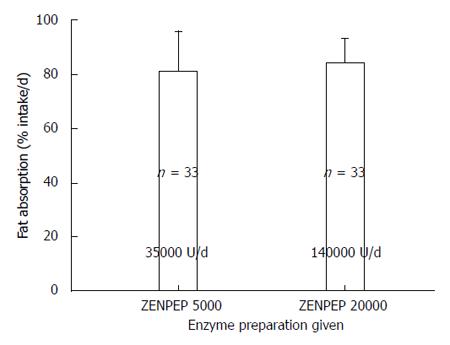
Figure 12 Effect of increasing the dose of enteric coated microbead therapy; seven 5000 USP unit tablets vs seven 20000 USP tablets (Zenpep®) on steatorrhea are shown (mean plus standard deviation).
Increasing the dosage 4-fold resulted in no significant improvement in steatorrhea and did not result in correction of steatorrhea[4].
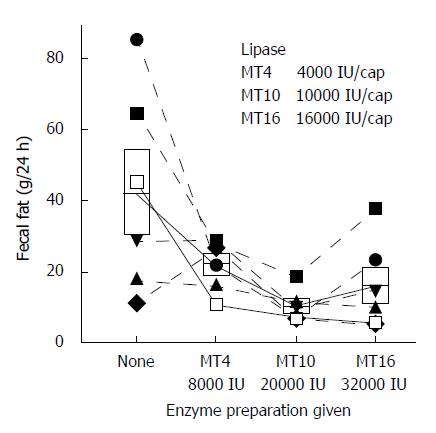
Figure 14 Effect of increasing the dosage of enteric coated microsphere preparation on fecal fat excretion is shown.
Sorted by treatment groups and individual data for all subjects. Increasing the dosage from 8000 IU 4-fold (24000 to 128000 USP units) failed to show a clear dose response effect or to reliably resolve steatorrhea. The box shows the mean and standard deviation for each group. From[65] with permission.
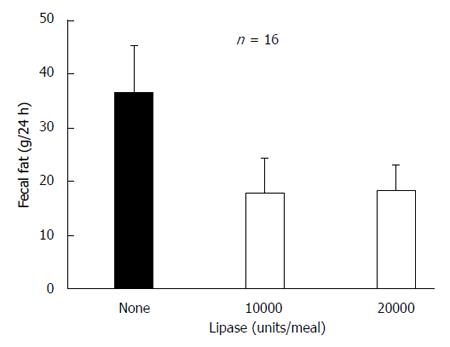
Figure 15 Effect of acid suppression with 60 mg of omeprazole on effectiveness of enzyme therapy with an enteric coated microsphere preparation (Pancrease®).
Comparison of 2 dosing regimens 10000 (2 capsule of 5000 USP Pancrease®) or 20000 USP (4 capsule 5000 USP Pancrease®) lipase units per meal. The results were the same and neither resolved the steatorrhea[82].
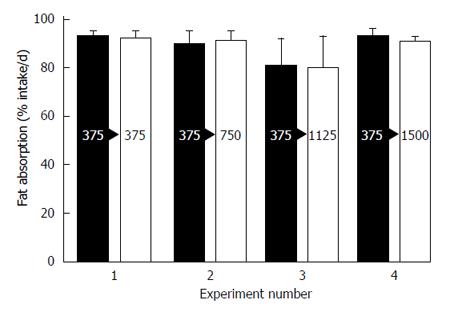
Figure 16 Data from 4 studies in children with cystic fibrosis comparing 375 USP lipase units/kg/meal to higher doses for the effect on steatorrhea.
The results did not show a consistent effect on increasing the lipase dosage of an enteric coated preparation (Pancreaze®). Mean plus standard deviation are shown[79].
- Citation: Trang T, Chan J, Graham DY. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21st century. World J Gastroenterol 2014; 20(33): 11467-11485
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11467