Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 7, 2014; 20(29): 9744-9758
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9744
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9744
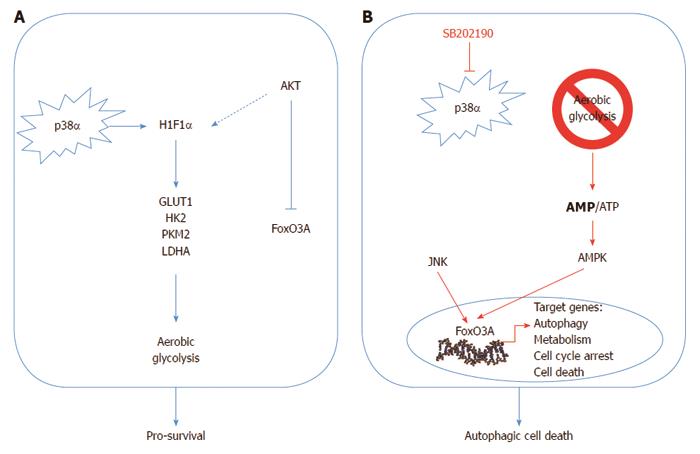
Figure 1 Schematic representation of the p38α involvement in colorectal cancer.
A: p38α is involved in the regulation of key metabolic cascades in colorectal cancer (CRC), sustaining HIF1α protein expression and the transcription of HIF1α target genes, such as GLUT1, HK2, PKM2 and LDHA; B: p38α blockade causes a significant decrease in the intracellular levels of ATP, which correlates with a time-dependent reduction of HIF1α protein levels and the consequent decrease in HIF1α target gene expression and phospho-activation of AMPK. AMPK activity is required for the nuclear accumulation of FoxO3A and the subsequent activation of FoxO3A target genes involved in autophagy, metabolism, cell cycle arrest and cell death, leading to autophagic cell death in CRC in vitro and in vivo. PI3K/Akt and JNK kinases regulate the nuclear/cytoplasmic shuttling of FoxO3A protein by phosphorylation.
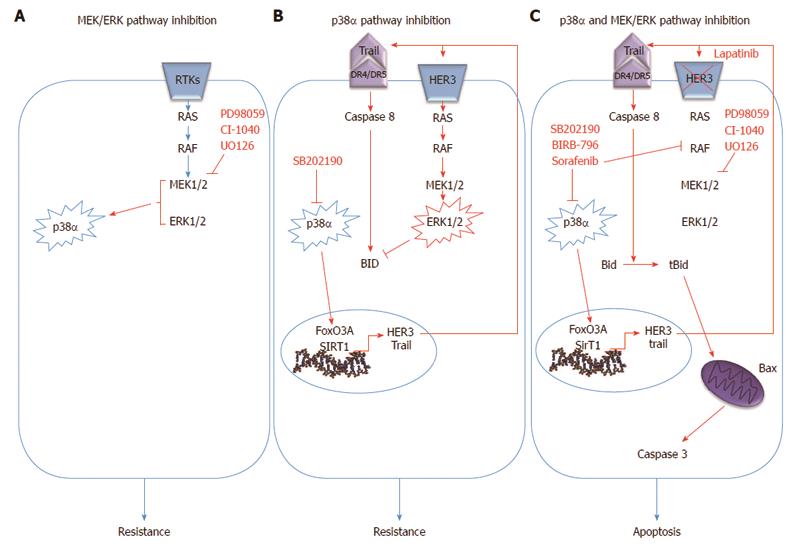
Figure 2 Schematic representation of the p38α/ERK cross-talk in colorectal cancer.
A: Inhibition of MEK1 triggers phospho-activation of the p38 MAPK pathway, indicating the existence of a p38α/ERK crosstalk in CRC cells; B: p38α inhibition induces increased expression of HER3, one of the receptor tyrosine kinases (RTK) of the EGF pathway, and this effect is dependent upon the activity of FoxO3A and its cofactor SIRT1. In turn, HER3 up-regulation leads to over-activation of the MEK/ERK pathway. Moreover, p38α inhibition leads to the rescue of a pro-apoptotic program driven by the extrinsic pathway through transcriptional up-regulation of TRAIL and activation of caspase-8; C: Concomitant MEK inhibition triggers Bax-dependent apoptosis by enabling signaling propagation through t-Bid and caspase 3. Bid phosphorylation at MAPK phosphorylation sites is induced by p38α inhibition and abrogated by concomitant inhibition of MEK1.
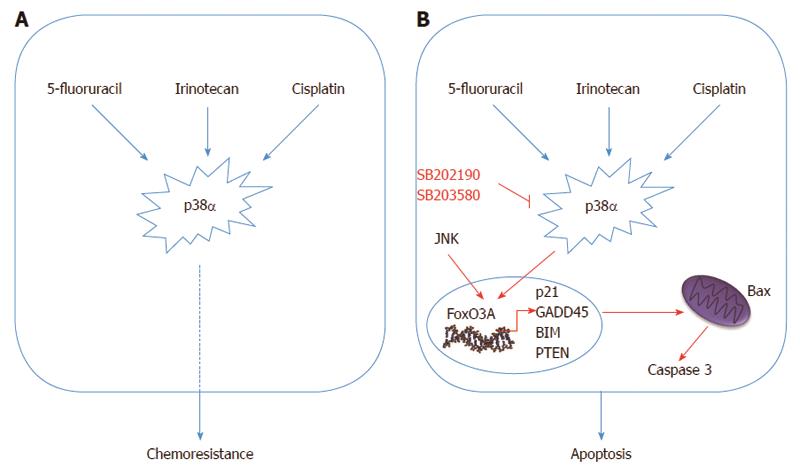
Figure 3 Schematic representation of the p38α as a mediator of resistance in colorectal cancer.
A: p38α is a mediator of resistance to irinotecan, 5-FU and cisplatin and its activation correlates with impaired response to therapy in colorectal cancer (CRC) patients; B: p38α pharmacological inhibition combined with chemotherapeutic agents decreases viability of cancer cells and strongly increases Bax-dependent apoptotic cell death by activating the tumor suppressor protein FoxO3A. FoxO3A activation up-regulates transcription of its target genes (p21, PTEN, Bim and GADD45), which forces both chemosensitive and chemoresistant CRC cells to undergo apoptosis.
- Citation: Grossi V, Peserico A, Tezil T, Simone C. p38α MAPK pathway: A key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol 2014; 20(29): 9744-9758
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9744.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9744