Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. May 21, 2014; 20(19): 5839-5848
Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5839
Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5839
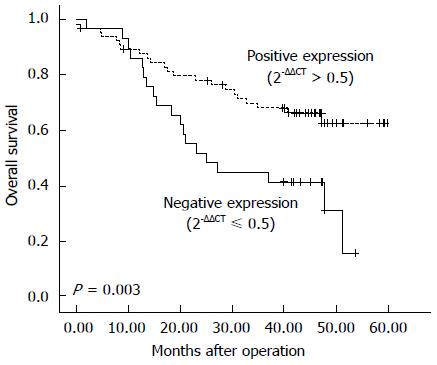
Figure 1 Kaplan-Meier estimates of overall survival by γ-catenin status in esophageal squamous cell carcinoma samples.
The Kaplan-Meier analyses of γ-catenin mRNA expression in 95 patients with esophageal squamous cell carcinoma illustrate that γ-catenin expression levels had an impact on the survival curve, with low γ-catenin (2-ΔΔCT≤ 0.5) indicating worse survival (P = 0.003).
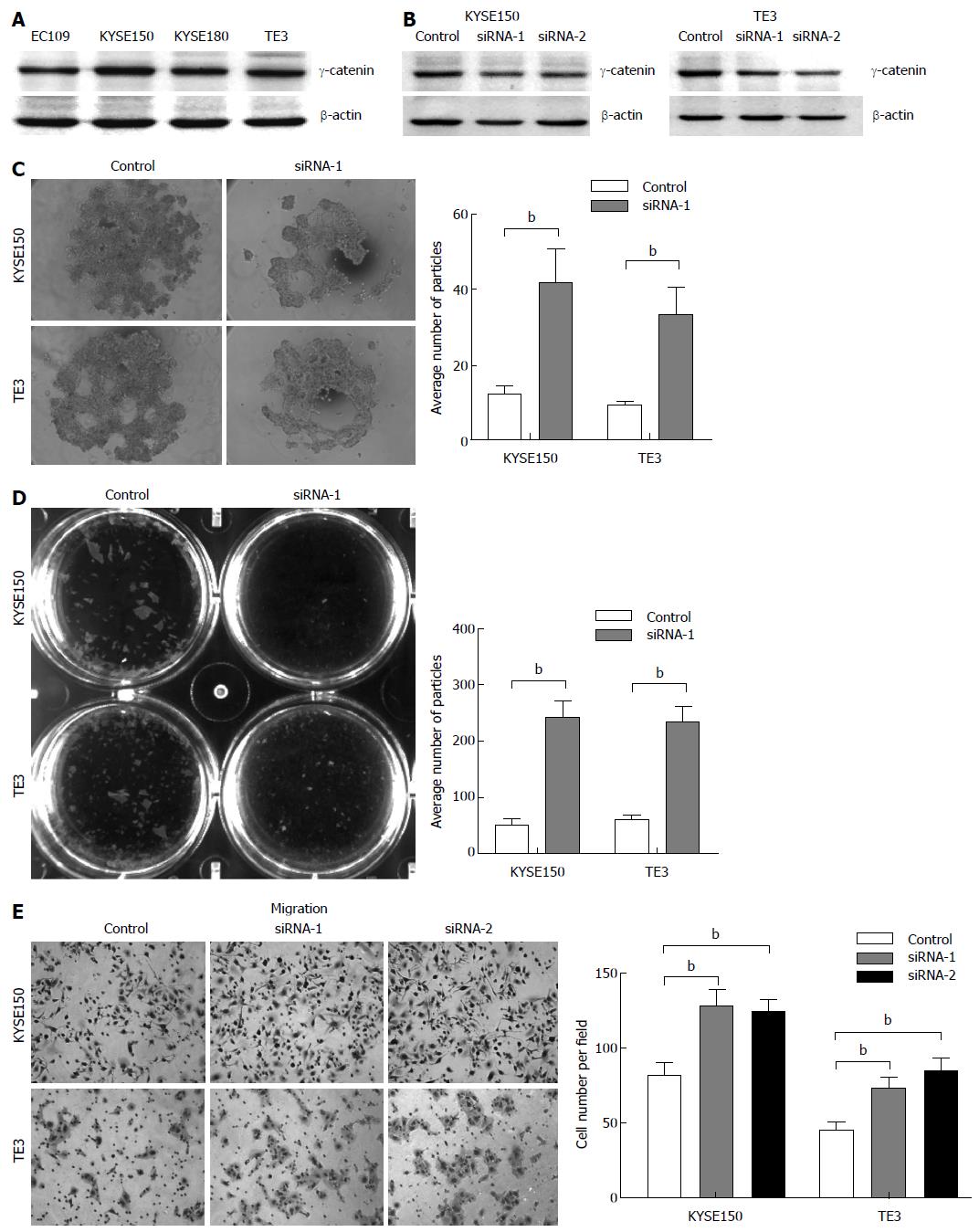
Figure 2 Alterations of cell-cell adhesion and cell migration exerted by RNAi-mediated knockdown of γ-catenin.
A: Representative Western blot of γ-catenin in 4 esophageal cancer cell lines; B: KYSE150 and TE3 cells were transfected with γ-catenin siRNAs (siRNA-1 and -2), or a negative control siRNA (control). Western blot analysis was used to show RNAi-mediated knockdown. Equal loading was ascertained using β-actin as an internal control; C: Hanging drop assay. Cells were seeded into hanging drop cultures and allowed to aggregate for 24 h. After trituration by passing the cell cluster 30 times through a 200 μL pipette tip, the degree of dissociation of the cell cluster was visualized by microscopy and quantified by manual counting under a dissecting microscope; D: Dispase-based dissociation assay. Cell monolayers were separated from culture dishes via incubation with dispase. Monolayers were transferred to 15 mL conical tubes containing 2 mL of phosphate buffered saline. After 50 inversions, the degree of fragmentation of the monolayers was observed. The dissociation assay was quantified by counting the number of total particles; E: Transwell assay was performed to determine cell migration. Migrated cells were fixed and stained, and representative fields were photographed. The cells were quantified in 10 random fields with a light microscope (× 200). The mean value was calculated from data obtained from three separate chambers. bP < 0.01 vs the control group.
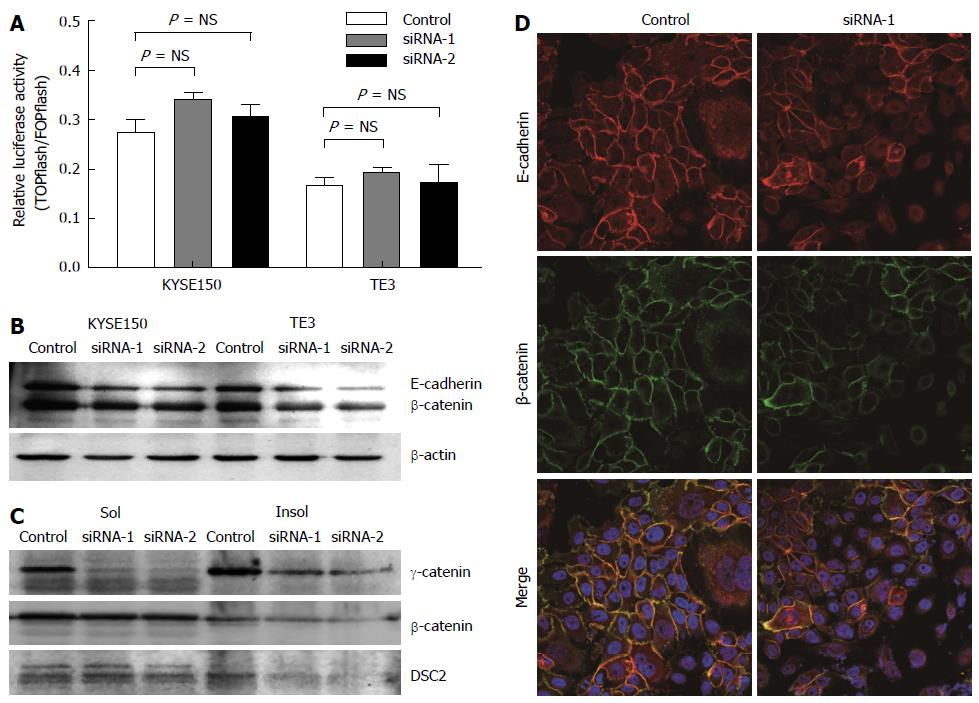
Figure 3 γ-catenin affects cell motility through cell-cell adhesion-dependent mechanisms.
A: TOPflash reporter activity in KYSE150 and TE3 cells. Cells were co-transfected with either TOPflash or FOPflash reporter plasmids along with siRNA-1/-2 or control siRNA. The level of β-catenin-dependent transcription was determined by TOPflash luciferase activity. FOPflash reporter plasmids containing mutant β-catenin/ TCF-binding sites were used as controls. Data represent the results of triplicate dishes from two independent experiments; B: Western blot analyses of E-cadherin and total β-catenin in γ-catenin-specific siRNA or control-transfected esophageal squamous cell carcinoma (ESCC) cells. β-actin served as a loading control; C: Western blot analyses of triton X-100-soluble and -insoluble γ-catenin, β-catenin and DSC2 proteins in γ-catenin-specific siRNA or control-transfected ESCC cells; D: The subcellular localizations of β-catenin and E-cadherin was examined using immunofluorescence analysis. Of note, knocking down γ-catenin expression caused reduced β-catenin and E-cadherin membrane localization.
- Citation: Fang WK, Liao LD, Gu W, Chen B, Wu ZY, Wu JY, Shen J, Xu LY, Li EM. Down-regulated γ-catenin expression is associated with tumor aggressiveness in esophageal cancer. World J Gastroenterol 2014; 20(19): 5839-5848
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5839.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5839