Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. May 7, 2014; 20(17): 4963-4971
Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.4963
Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.4963
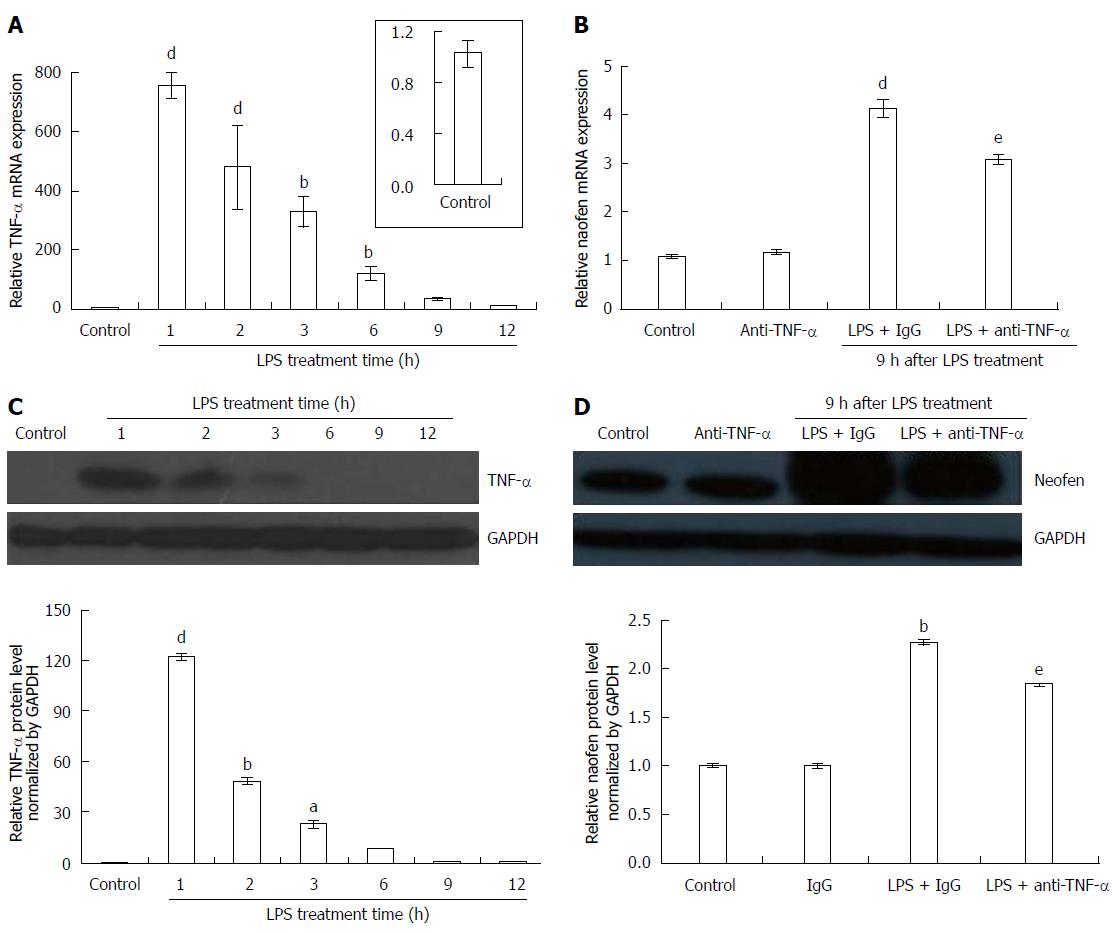
Figure 1 Changes in tumor necrosis factor-α and naofen expression in the liver of lipopolysaccharide-treated rats.
Rats were intravenously injected with saline + IgG (control), LPS (500 μg/kg) + IgG (2 mg/kg) or LPS (500 μg/kg) + anti-TNF-α (2 mg/kg), and livers were removed to evaluate expression of TNF-α and naofen. A: Time course of the effects of LPS on TNF-α mRNA expression; B: Effect of anti-TNF-α antibody on naofen mRNA expression at 9 h after LPS injection; total RNA was extracted using TRIzol reagent and relative mRNA was quantified using qPCR (n = 10). Tissue lysates were examined by Western blot; C: TNF-α protein expression; D: Naofen protein expression (n = 6). Results are presented as ratios of target mRNA or protein normalized to internal GAPDH. aP < 0.05, bP < 0.01 and dP < 0.001 vs control; eP < 0.05 vs LPS + IgG. TNF-α: Tumor necrosis factor-α; LPS: Lipopolysaccharide.
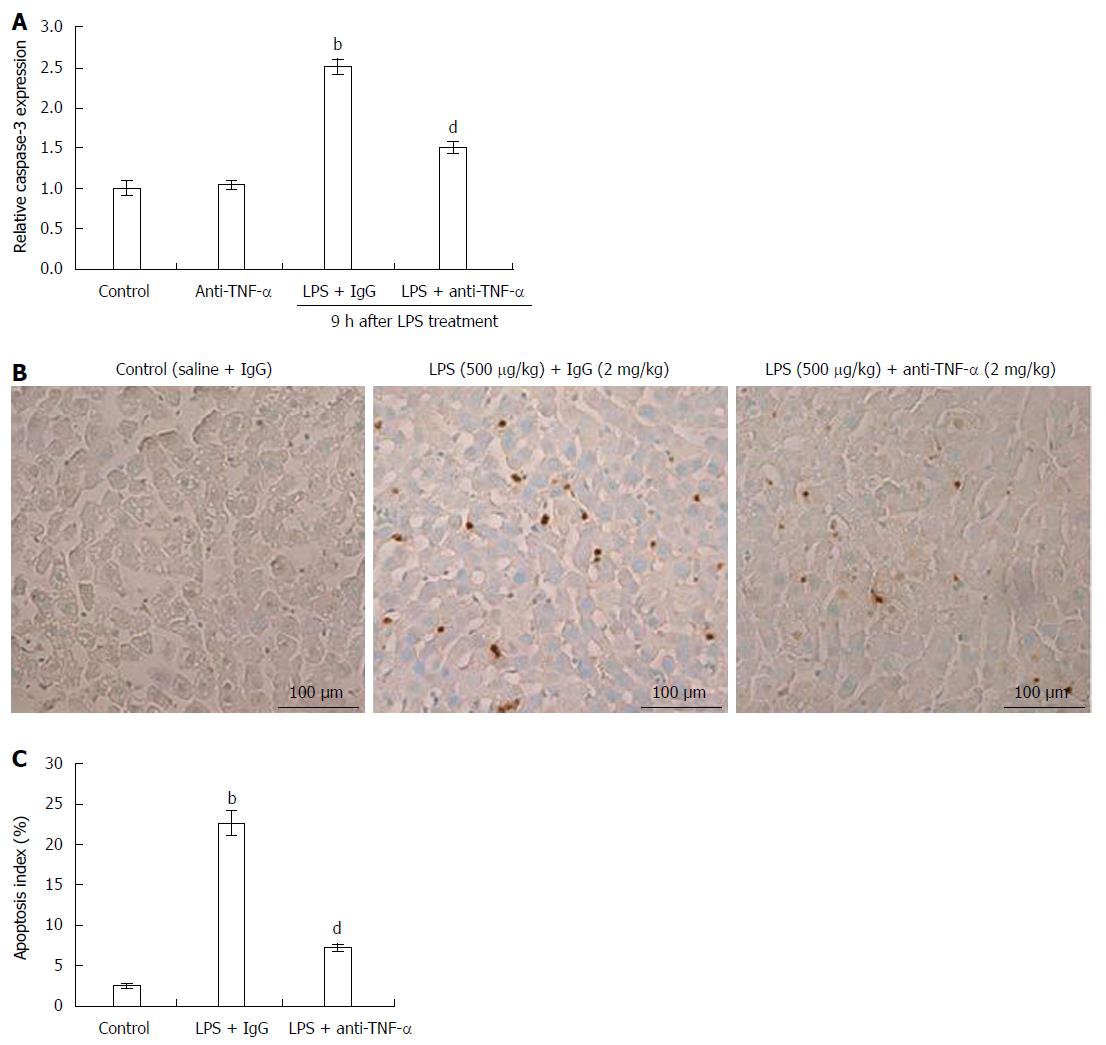
Figure 2 Effects of anti-tumor necrosis factor-α antibody on caspase-3 activation and apoptosis.
Rats were treated as described above. A: Caspase-3 activation. Livers were lysed in lysis buffer and caspase-3 activation was measured. bP < 0.01 vs controls; dP < 0.01 vs LPS + IgG (n = 10); B: Liver apoptosis was examined using dUTP nick end labeling assay in rats 9 h after injection with control (saline + IgG), LPS + IgG or LPS + anti-TNF-α. Bar: 100 μm; C: Apoptosis index in the different groups. bP < 0.01 vs control; dP < 0.01 vs LPS + IgG (n = 10). TNF-α: Tumor necrosis factor-α; LPS: Lipopolysaccharide.
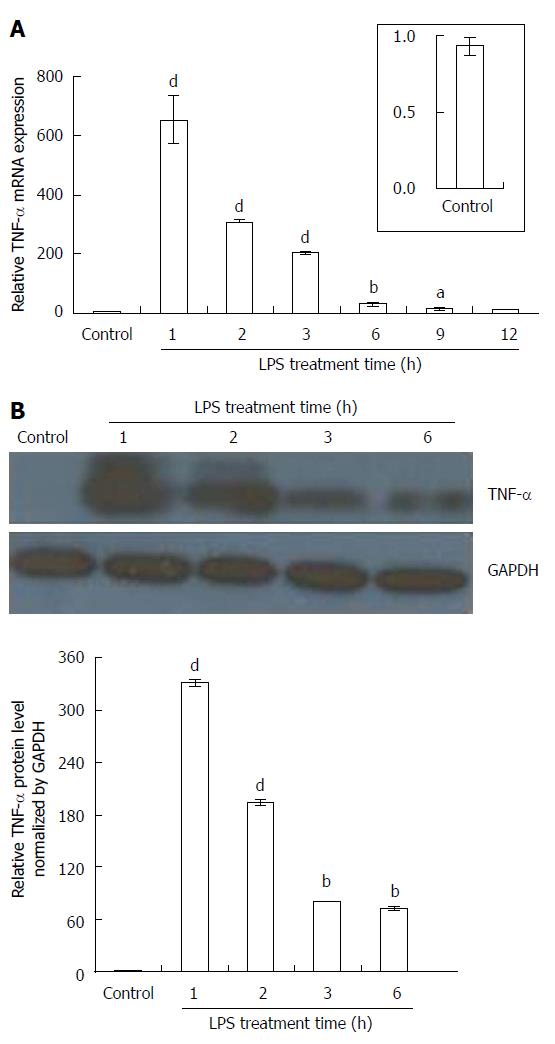
Figure 3 Effects of exposure period of Kupffer cells to lipopolysaccharide on tumor necrosis factor-α production.
Primary Kupffer cells (KCs) were separated from rat livers using collagenase perfusion. After 24 h of incubation, KCs were incubated with lipopolysaccharide (LPS) (100 ng/mL). A: Tumor necrosis factor (TNF)-α mRNA was quantified using qPCR (n = 6); B: Immunoblotting assay for TNF-α. KC lysates were analyzed with TNF-α antibody (n = 6). GAPDH was used as an internal control. aP < 0.05, bP < 0.01 and dP < 0.001 vs controls (without LPS treatment).
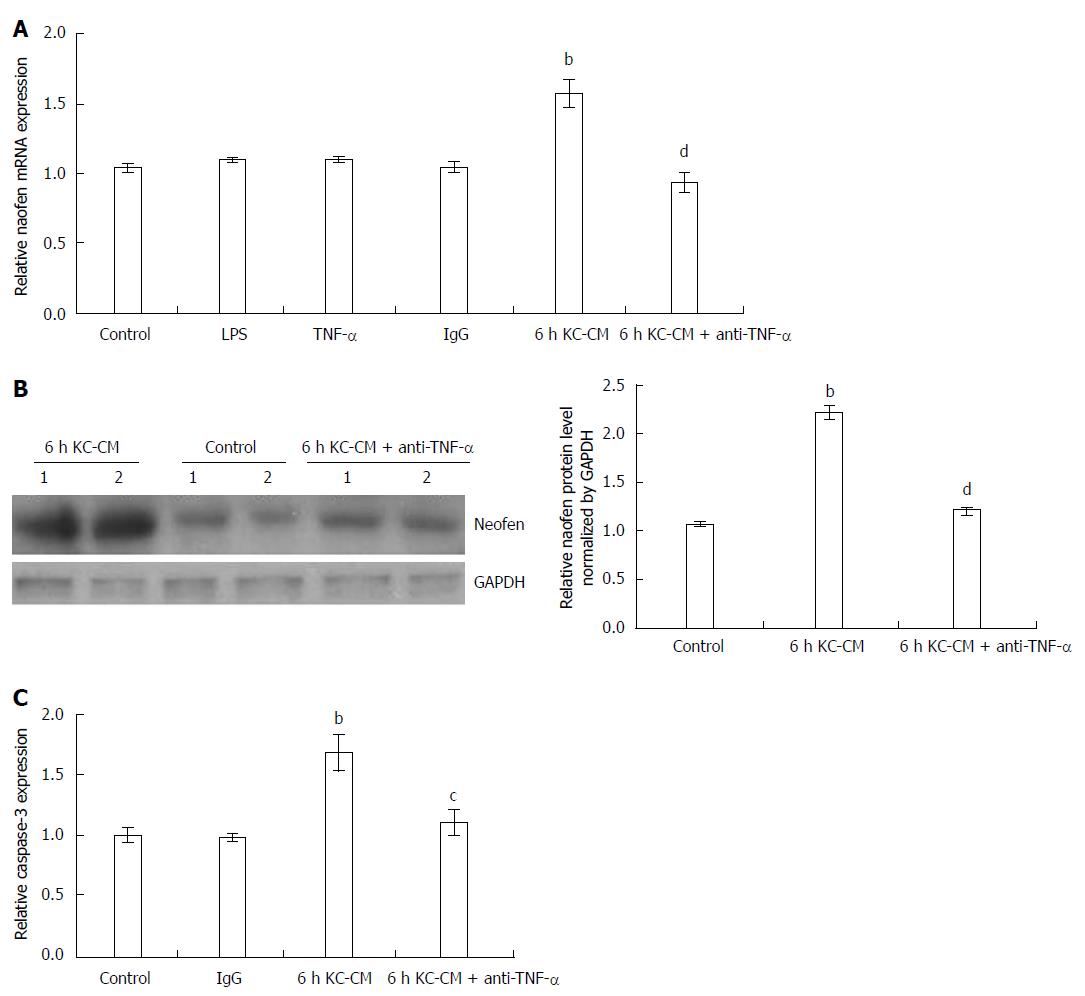
Figure 4 Effect of Kupffer cell-conditioned medium on naofen mRNA expression and caspase-3 activation.
Primary Kupffer cell (KCs) and hepatocytes were separated as described above, and KC-CM was obtained by incubating KCs with LPS (100 ng/mL) for 6 h. Anti-TNF-α antibody (500 ng/mL) was added to KC-CM treated with LPS for 6 h and incubated at 37 °C for 1 h (anti-TNF-α + 6 h KC-CM). Hepatocytes were incubated with LPS (100 ng/mL), TNF-α (10 ng/mL), IgG (500 ng/mL), 6 h KC-CM and anti-TNF-α + 6 h KC-CM for 12 h, respectively. A: Naofen mRNA in hepatocytes was measured with qPCR and GAPDH was used as an internal control (n = 6); B: Immunoblotting assay for naofen. Hepatocyte lysates were analyzed with naofen antibody (n = 6); C: Caspase-3 activation was also measured (n = 6). bP < 0.01 vs controls; cP < 0.05 and dP < 0.01 vs 6 h KC-CM. TNF-α: Tumor necrosis factor-α; LPS: Lipopolysaccharide; KC-CM: Kupffer cell-conditioned medium.
- Citation: Fan JH, Feng GG, Huang L, Tang GD, Jiang HX, Xu J. Naofen promotes TNF-α-mediated apoptosis of hepatocytes by activating caspase-3 in lipopolysaccharide-treated rats. World J Gastroenterol 2014; 20(17): 4963-4971
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/4963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.4963