Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. May 28, 2013; 19(20): 3069-3076
Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3069
Published online May 28, 2013. doi: 10.3748/wjg.v19.i20.3069
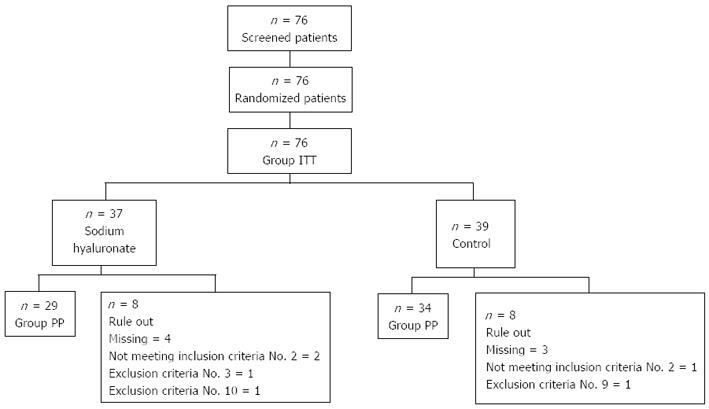
Figure 1 Trial profile.
Inclusion criteria No. 2, 5 mm ≤ adenoma or adenocarcinoma ≤ 20 mm; exclusion criteria No. 3, undifferentiated adenocarcinoma; exclusion criteria No. 9, severe functional abnormalities of cardiovascular system; exclusion criteria No. 10, concomitant medication with systemic prednisolone, anticancer agents or immunosuppressive agents. ITT: Intention to treat; PP: Per protocol.
- Citation: Kim YD, Lee J, Cho JY, Kim SW, Kim SH, Cho YK, Jang JS, Han JS, Cho JY. Efficacy and safety of 0.4 percent sodium hyaluronate for endoscopic submucosal dissection of gastric neoplasms. World J Gastroenterol 2013; 19(20): 3069-3076
- URL: https://www.wjgnet.com/1007-9327/full/v19/i20/3069.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i20.3069