Copyright
©2012 Baishideng Publishing Group Co.
World J Gastroenterol. May 7, 2012; 18(17): 2053-2060
Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2053
Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2053
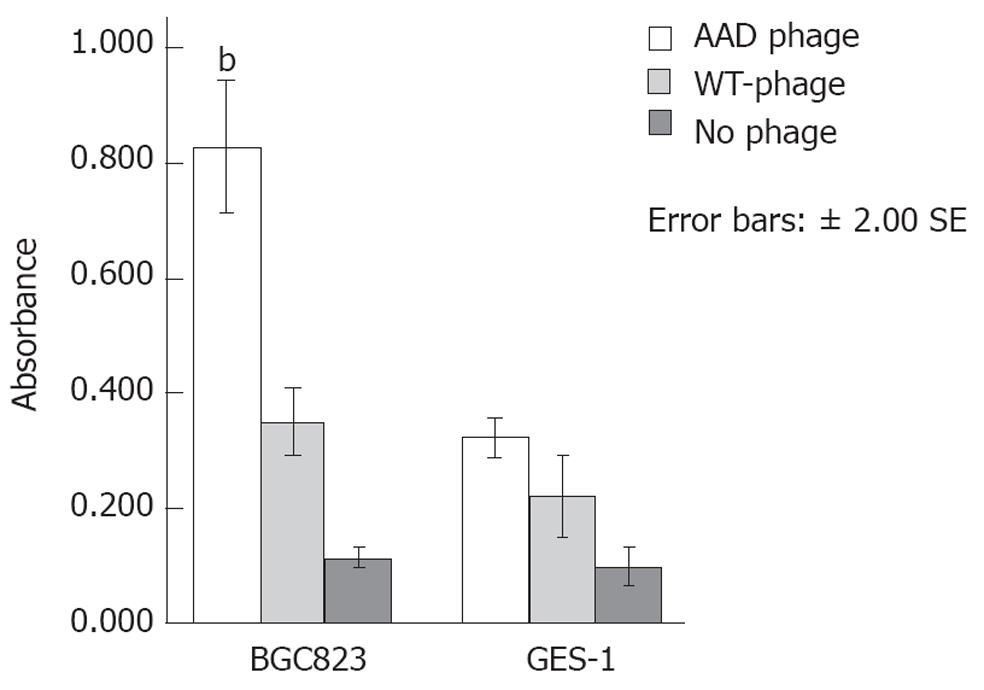
Figure 1 Preferential phage-binding to BGE823 and GES-1 cells.
Phage capture enzyme-linked immunosorbent assay revealed a greater optical density at binding sites of AADNAKTKSFPV (AAD) phage to BGE823 cells compared with that of wild type phage (bP < 0.01) or no phage. No significant difference was found in binding of AAD phage to the control cells. WT: Wild type.
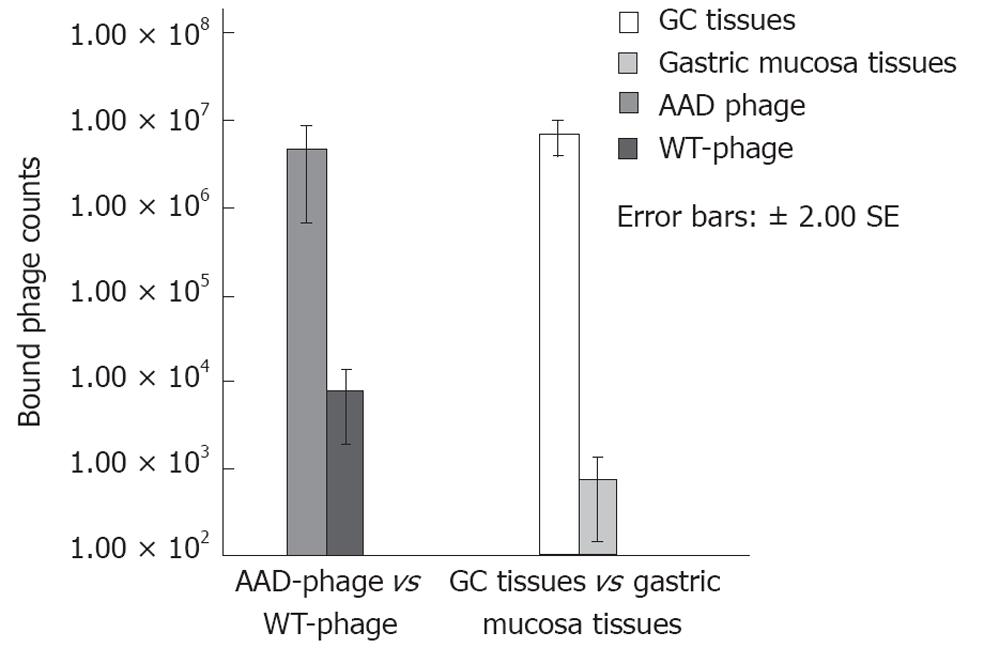
Figure 2 Phage binding affinity.
AADNAKTKSFPV (AAD) phage showed an about 615 times higher binding efficiency in gastric cancer (GC) tissues than wild type (WT)-phage, and the binding of AAD phage was about 591 times greater in GC tissues than in gastric mucosa.
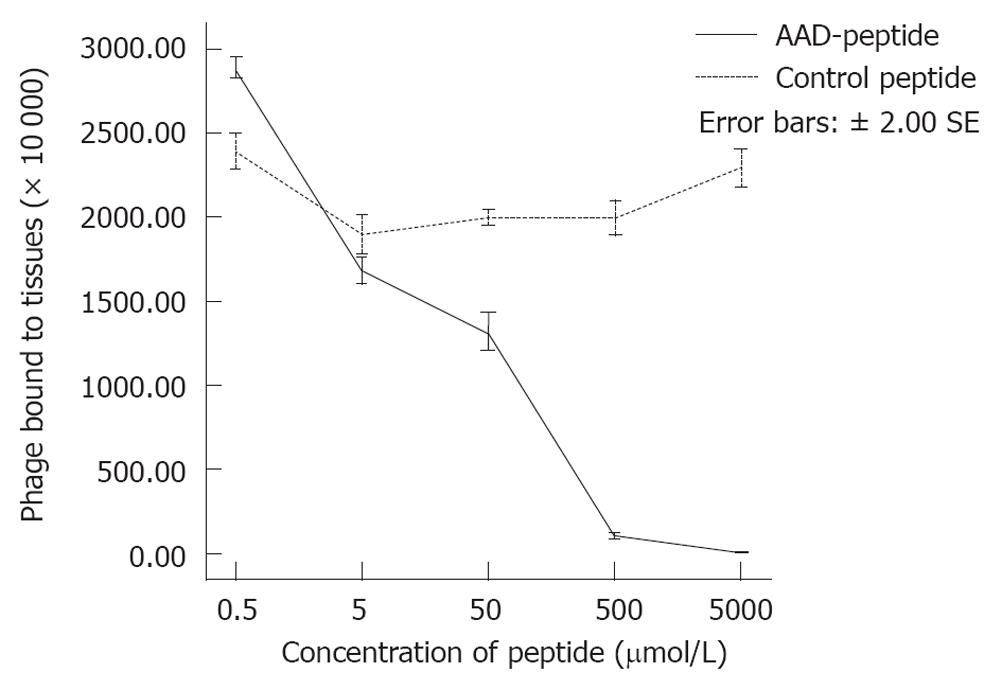
Figure 3 Competition binding assay.
Binding of AADNAKTKSFPV (AAD) phage to gastric cancer tissues is reduced by competition with increasing concentrations of AAD peptide (P < 0.01) in a dose-dependent manner. The addition of the control peptide at concentrations of 0.5, 5, 50, 500 and 5000 μmol revealed no competitive inhibition.
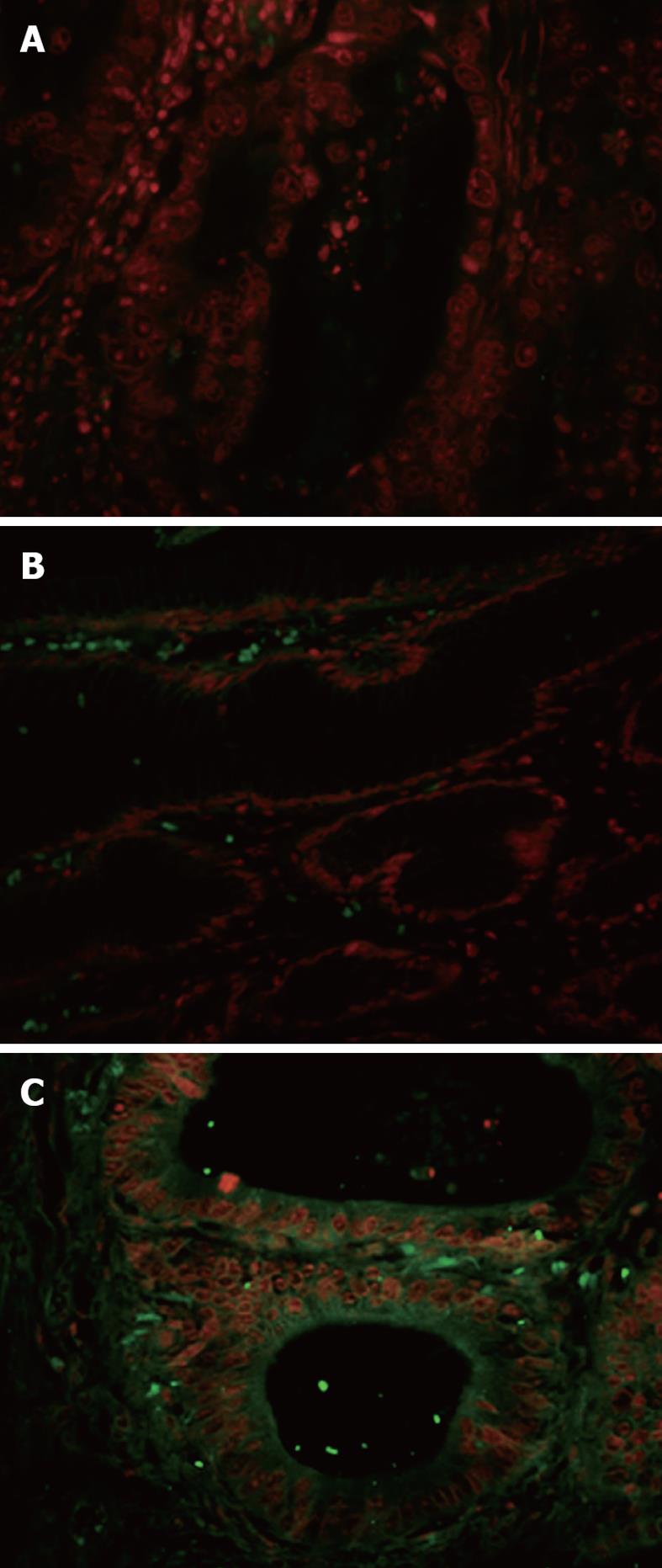
Figure 4 Immunofluorescence analysis of fluorescein isothiocyanate-conjugated AADNAKTKSFPV binding to human gastric cancer tissues.
Frozen sections for biopanning were incubated with fluorescein isothiocyanate-conjugated AADNAKTKSFPV (AAD); scrambled peptide PAKFKAANSDVT was used as the control. Immunofluorescence stain with FITC-conjugated AAD showed selective signals (green) located in tumor membrane, cytoplasm (C), but no binding to normal gastric mucosae (B). As a control, the scramble peptide displayed no signals in tumor tissues (A).
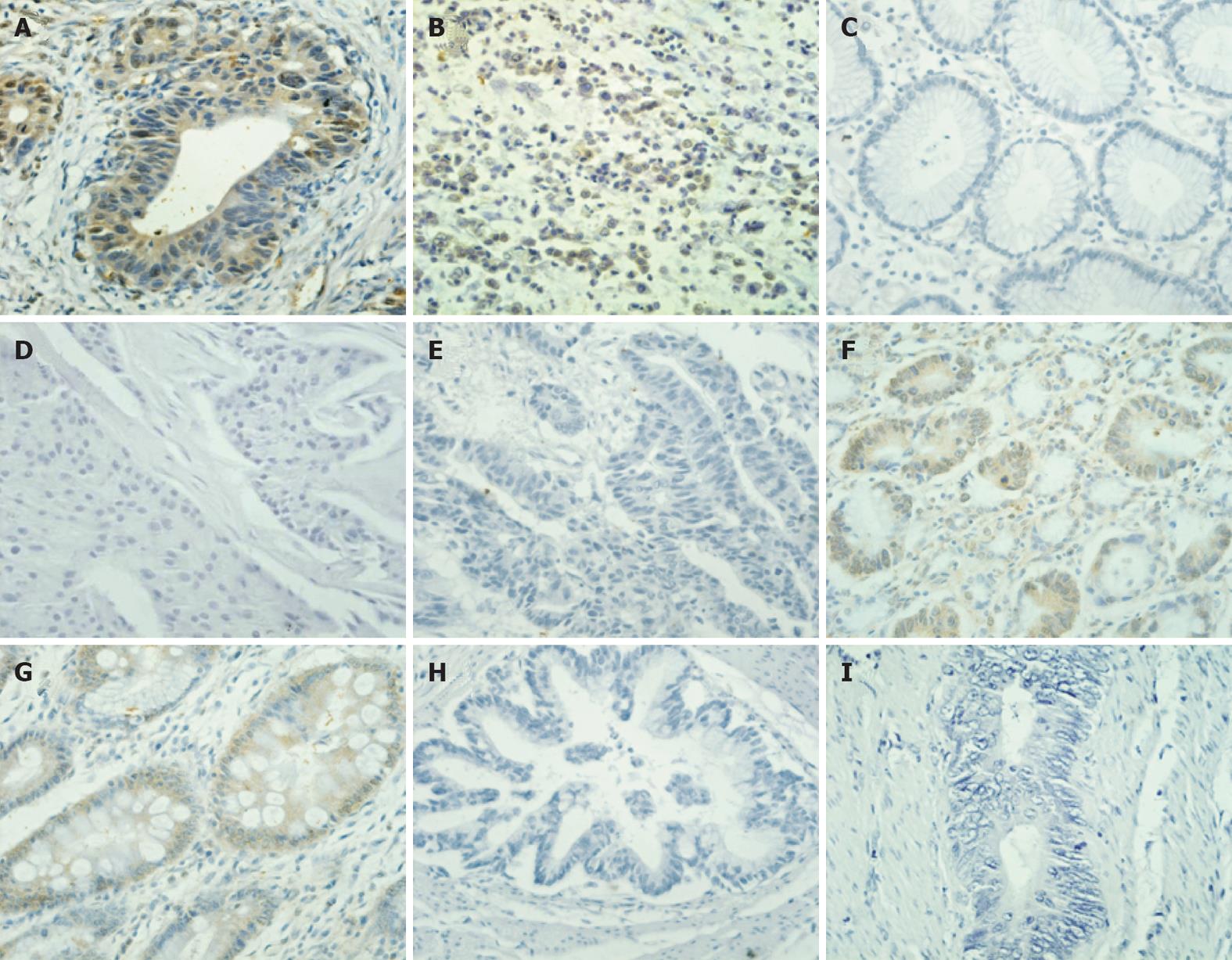
Figure 5 Binding analysis of biotin-AADNAKTKSFPV by immunohistochemistry.
The results demonstrate that biotin showed a specific binding affinity to gastric cancer (GC) (A: Intestinal; B: Diffuse). In contrast, no positive staining was observed in gastric mucosae (C). In addition, peptide AADNAKTKSFPV (AAD) did not bind to breast cancer (D) or colon cancer (E), suggesting that the AAD peptide is specific to GC. A small amount of binding of the peptide AAD to the gastric mucosa dysplasia (F) and intestinal metaplasia (G) was also observed. The negative results were also obtained when GC tissues were stained with the biotin-conjugated scramble peptide (H) or phosphate-buffered saline (I).
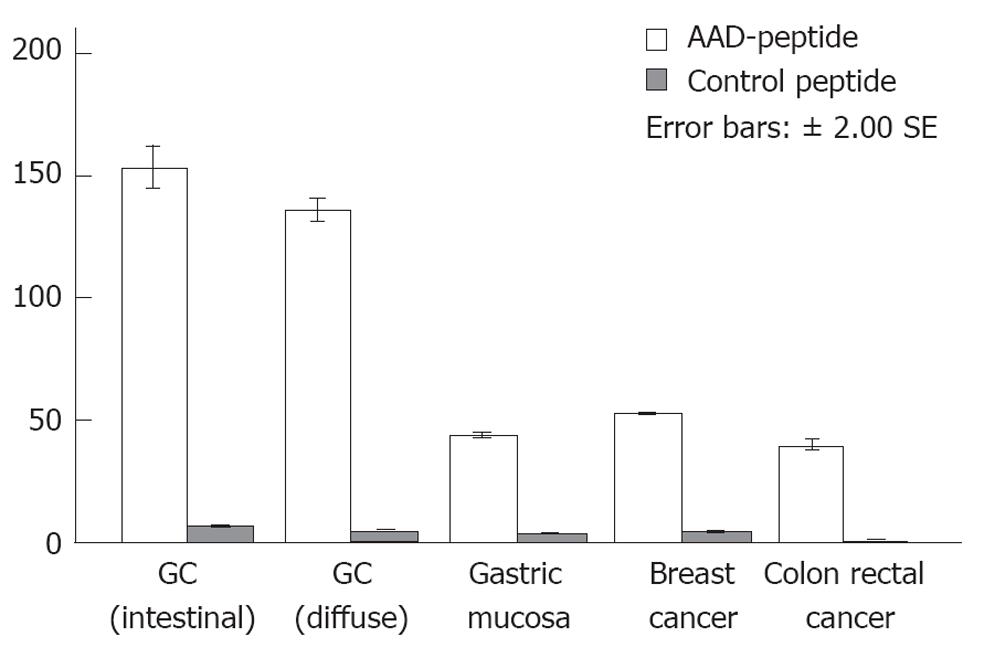
Figure 6 Semi-quantitative image analysis.
AADNAKTKSFPV (AAD) peptide is statistically significant for gastric cancer as compared with other histological classifications and control peptide. GC: Gastric cancer.
- Citation: Zhang WJ, Sui YX, Budha A, Zheng JB, Sun XJ, Hou YC, Wang TD, Lu SY. Affinity peptide developed by phage display selection for targeting gastric cancer. World J Gastroenterol 2012; 18(17): 2053-2060
- URL: https://www.wjgnet.com/1007-9327/full/v18/i17/2053.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i17.2053