Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 7, 2011; 17(5): 557-566
Published online Feb 7, 2011. doi: 10.3748/wjg.v17.i5.557
Published online Feb 7, 2011. doi: 10.3748/wjg.v17.i5.557
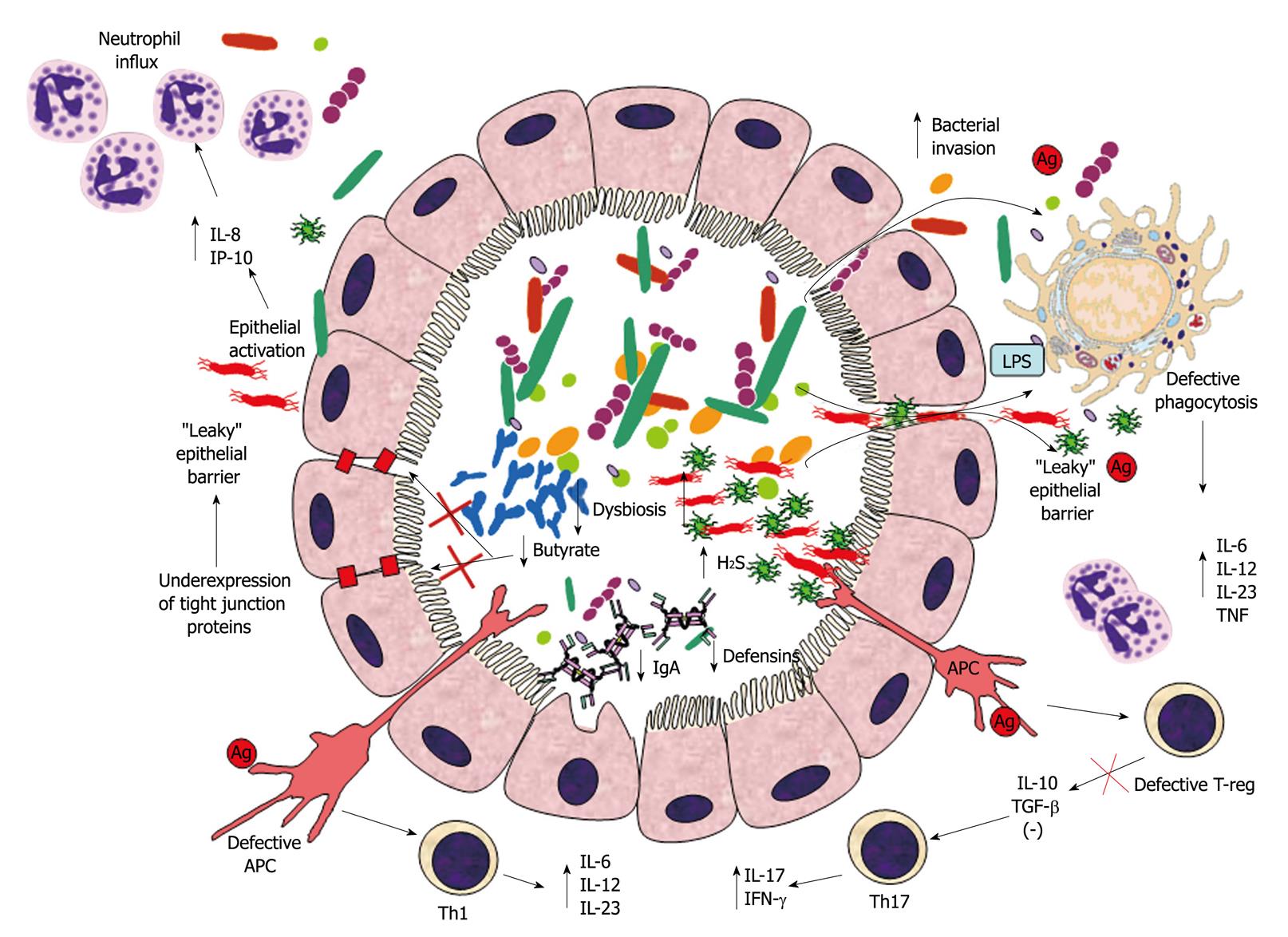
Figure 1 Suggested mechanism of inflammatory bowel disease pathogenesis.
Intestinal dysbiosis in inflammatory bowel disease (IBD) consists of decreased prevalence of putative beneficial bacteria (e.g. bifidobacteria) and concomitant increase in detrimental bacterial (e.g. sulphate-reducing bacteria). This microbial imbalance causes reduced intraluminal levels of butyrate (because of decreased production through fermentation and decreased utilization due to increased H2S levels), thus contributing to down-regulation of epithelial tight junction protein expression and increased epithelial permeability. Epithelial barrier dysfunction brings about increased bacterial translocation through the lamina propria, which is worsened by decreased luminal IgA and defensin concentrations. Killing of bacteria reaching the lamina propria through the “leaky” epithelium is also impaired by a genetically predisposed defective phagocytosis by macrophages. Ineffective bacterial clearance leads to excessive toll-like receptor (TLR) stimulation, secretion of pro-inflammatory cytokines and activation of innate and T-cell mediated immune responses. The disrupted mechanism of tolerance in epithelial cells and antigen presenting cells (APC) amplifies innate immune cell recruitment (i.e. neutrophils). Additionally, defective T-reg and APC cause excessive T-cell response (Th1 and Th17), with consequential intensification of the inflammatory response and granulomatous reaction. IL: Interleukin; IFN-γ: Interferon-γ; TNF: Tumor necrosis factor; TGF-β: Transforming growth factor-β; LPS: Lipopolysaccharide.
- Citation: Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J Gastroenterol 2011; 17(5): 557-566
- URL: https://www.wjgnet.com/1007-9327/full/v17/i5/557.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i5.557