Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Oct 28, 2011; 17(40): 4488-4495
Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4488
Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4488
Figure 1 Increased expression of αV integrin in response to gastrin in Panc-1 cells.
A: Results of probing a 96 genes array with samples from unstimulated Panc-1 cells (control) or cells stimulated with 100 nmol/L of gastrin for 48 h; B: Real time polymerase chain reaction (PCR) analysis of αV integrin mRNA expression in Panc-1 cells. Cells were treated or not with gastrin for the time indicated. Total RNA was isolated and αV integrin mRNA expression was determined by real time PCR as described Materials and Methods; C, D: Expression of αV integrin protein was examined by Western analysis following treatment of the cells with gastrin for 24 h. Blots were also probed with an antibody against GAPDH to ensure equal loading of proteins. Representative data from 3 experiments are shown. Quantifications of three experiments are presented as mean ± SE. Significance was accepted at P≤ 0.05, aP < 0.05, bP < 0.01.
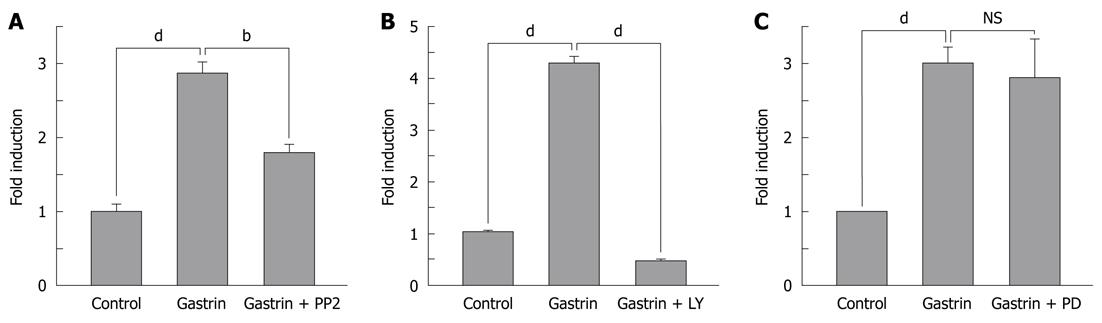
Figure 2 Signalling pathways involved in αV integrin expression stimulated by gastrin.
Cells were pretreated for 30 min with (B) a specific Pi3K inhibitor (LY294002, 20 μmol/L), (A) a Src-kinase inhibitor (PP2, 30 μmol/L) or (C) a MEK inhibitor (PD PD098059, 20 μmol/L) prior to gastrin stimulation. After 24 h, total RNA was isolated. Quantitative real-time polymerase chain reaction was performed as described in Materials and Methods. Quantifications of three experiments are presented as mean ± SE. Significance was accepted at P≤ 0.05, bP < 0.01, dP < 0.001. NS: Not significant.
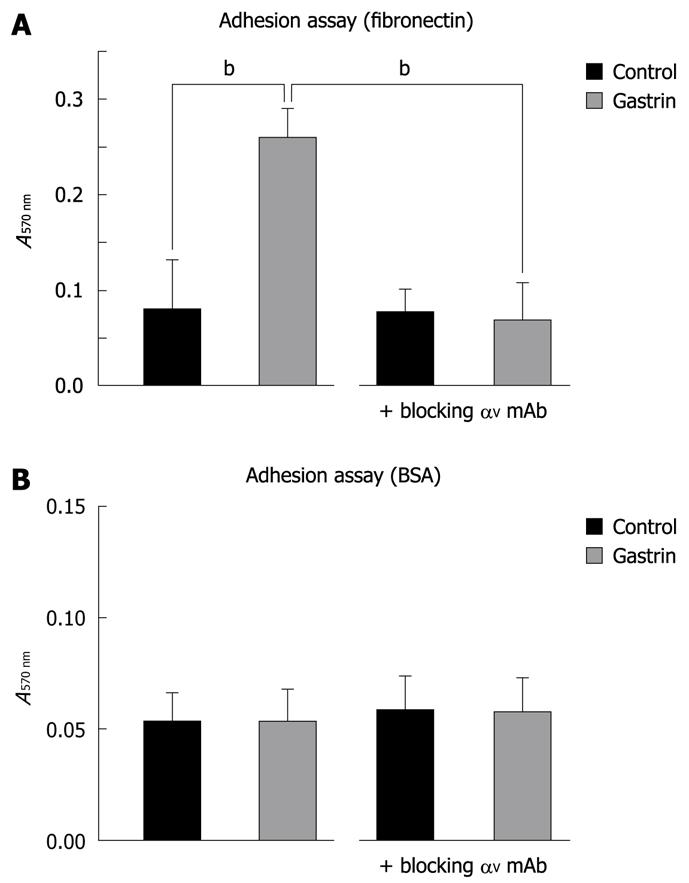
Figure 3 Effect of gastrin on Panc-1 cell adhesion.
Cells were added to fibronectin-coated (A) or non-coated (BSA alone) (B) 96-wells for 2 h in the presence or absence of gastrin. Adherent cells were fixed and stained with crystal violet as described in Materials and Methods. After solubilisation, absorbance was measured at 570 nm. When indicated, Panc-1 cells were pre-incubated, with a blocking αV mAb for 30 min prior to gastrin treatment for 2 h. Quantifications of three experiments are presented as mean ± SE. Significance was accepted at P≤ 0.05. bP < 0.01. BSA: Bovine serum albumin.
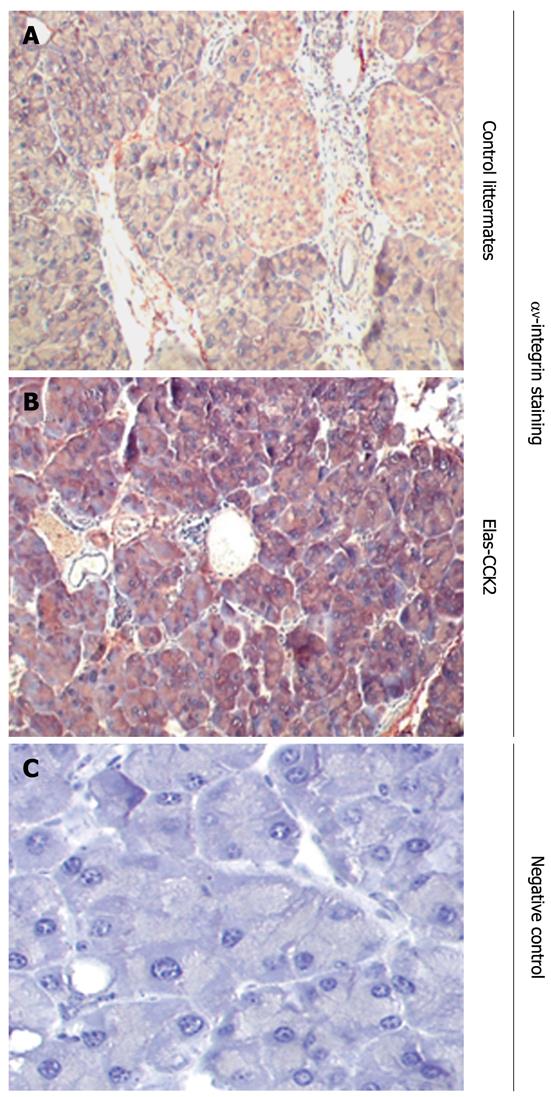
Figure 4 Overexpression of αV integrin in the pancreas of Elas-CCK2 mice.
Immunohistochemistry analysis of paraffin-embedded pancreatic tissues from Elas-CCK2 mice and control littermates were performed using antibodies specific for αV integrin (A, B). Representative data from 3 experiments (3 different animals in each group) are shown. A negative control without secondary antibody was also included (C).
- Citation: Cayrol C, Bertrand C, Kowalski-Chauvel A, Daulhac L, Cohen-Jonathan-Moyal E, Ferrand A, Seva C. αV integrin: A new gastrin target in human pancreatic cancer cells. World J Gastroenterol 2011; 17(40): 4488-4495
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4488.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4488