Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Oct 14, 2011; 17(38): 4321-4333
Published online Oct 14, 2011. doi: 10.3748/wjg.v17.i38.4321
Published online Oct 14, 2011. doi: 10.3748/wjg.v17.i38.4321
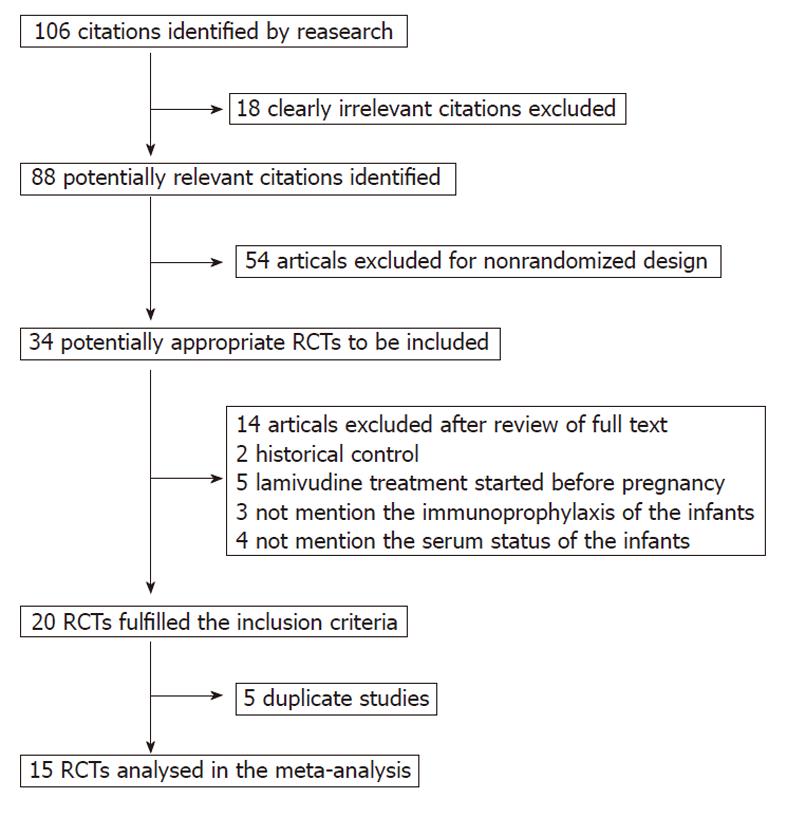
Figure 1 Flow chart of study recruitment.
RCTs: Randomized controlled clinical trials.
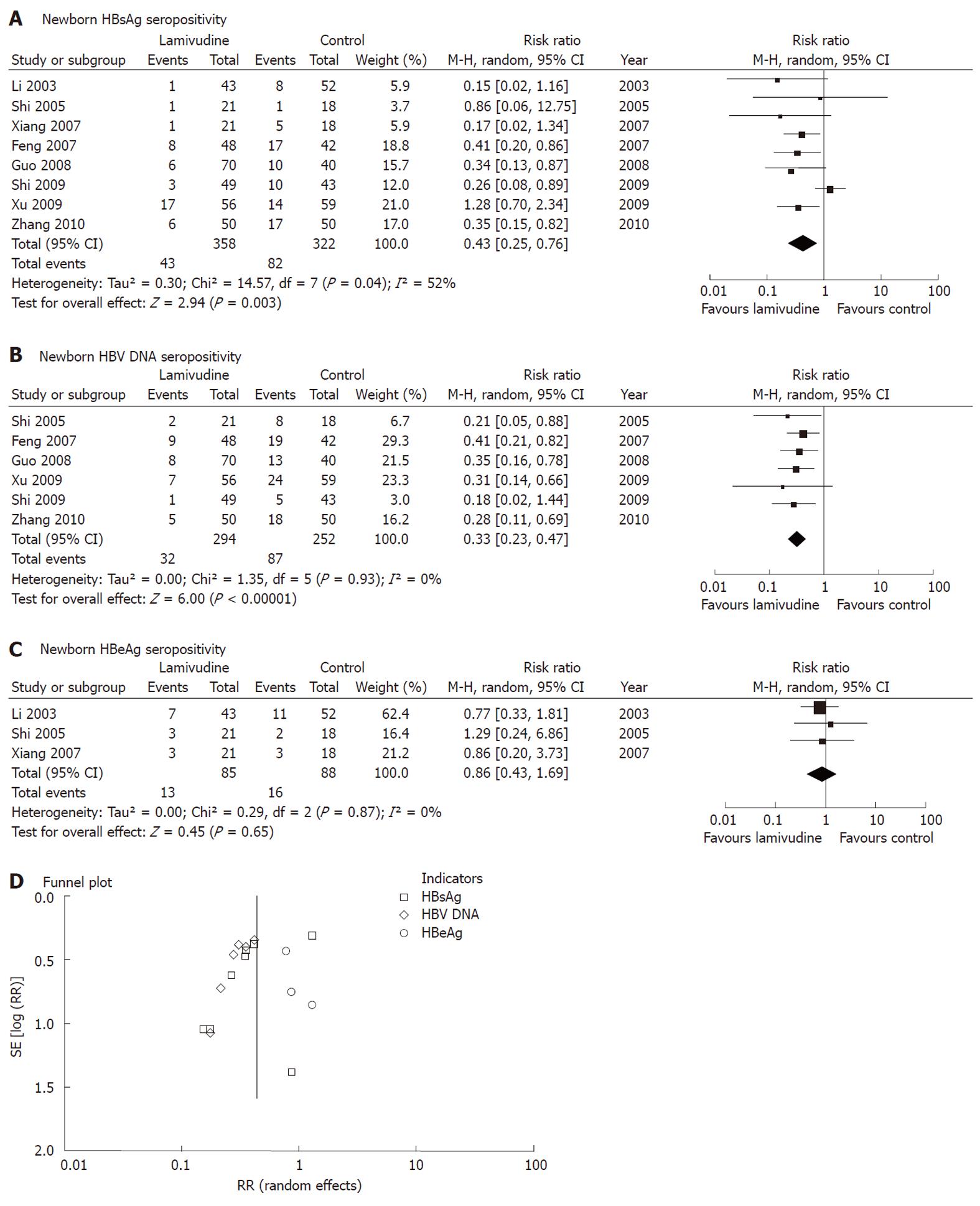
Figure 2 Effect of lamivudine treatment vs control (placebo or no intervention) on interruption of hepatitis B virus mother-to-child transmission as indicated by newborn serum hepatitis B surface antigen or hepatitis B virus DNA.
Vertical line indicates no difference between compared treatment. Horizontal lines show 95% CIs. Squares indicate point estimates, and the size of the squares indicates the weight of each study in the meta-analysis. HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; M-H random: Mantel-Haenszel random-effects model; CI: Confidence interval; HBV: Hepatitis B virus; RR: Risk ratio.
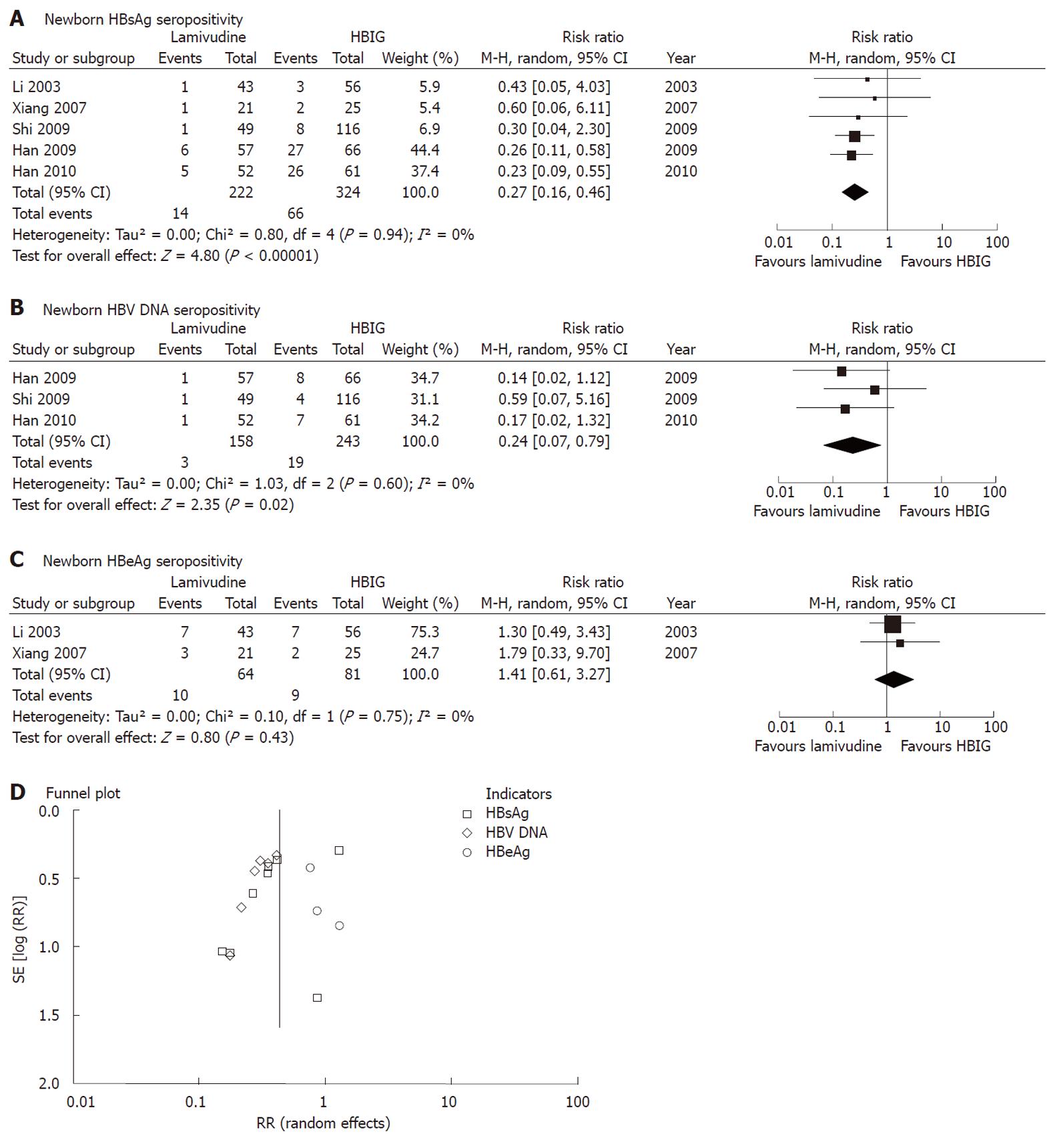
Figure 3 Lamivudine treatment vs hepatitis B immunoglobulin in interruption of hepatitis B virus mother-to-child transmission as indicated by newborn serum hepatitis B surface antigen or hepatitis B virus DNA.
CI: Confidence interval; HBIG: Hepatitis B immunoglobulin; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; HBV: Hepatitis B virus; M-H random: Mantel-Haenszel random-effects model; RR: Risk ratio.
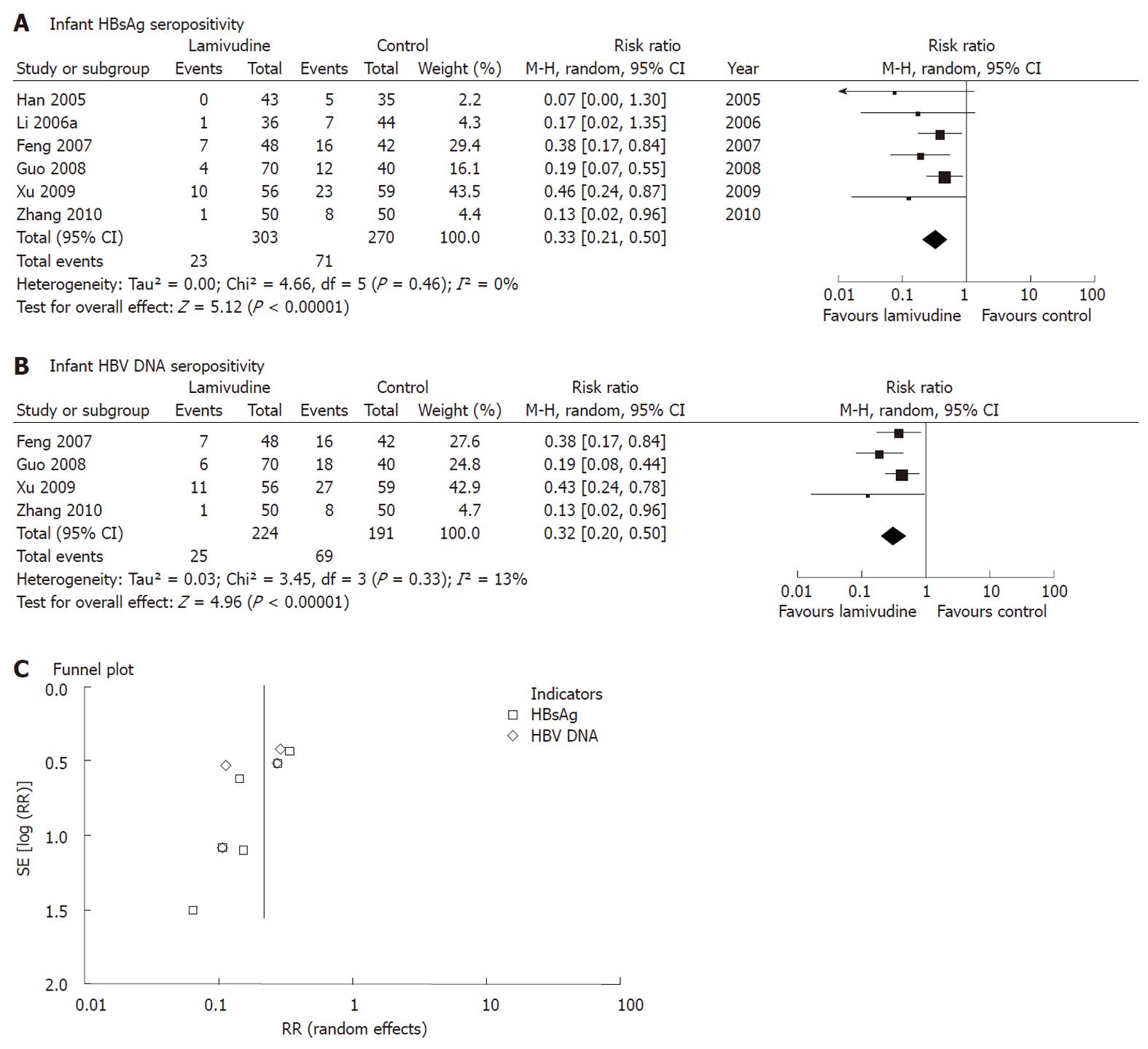
Figure 4 Effects of lamivudine vs control (placebo or no intervention) on interruption of hepatitis B virus mother-to-child transmission as indicated by serum hepatitis B surface antigen or hepatitis B virus DNA of infants 6-12 mo after birth.
Vertical line indicates no difference between compared treatment. Horizontal lines show 95% CIs. Squares indicate point estimates, and the size of the squares indicates the weight of each study in the meta-analysis. HBsAg: Hepatitis B surface antigen; M-H random: Mantel-Haenszel random-effects model; CI: Confidence interval; HBV: Hepatitis B virus; RR: Risk ratio.
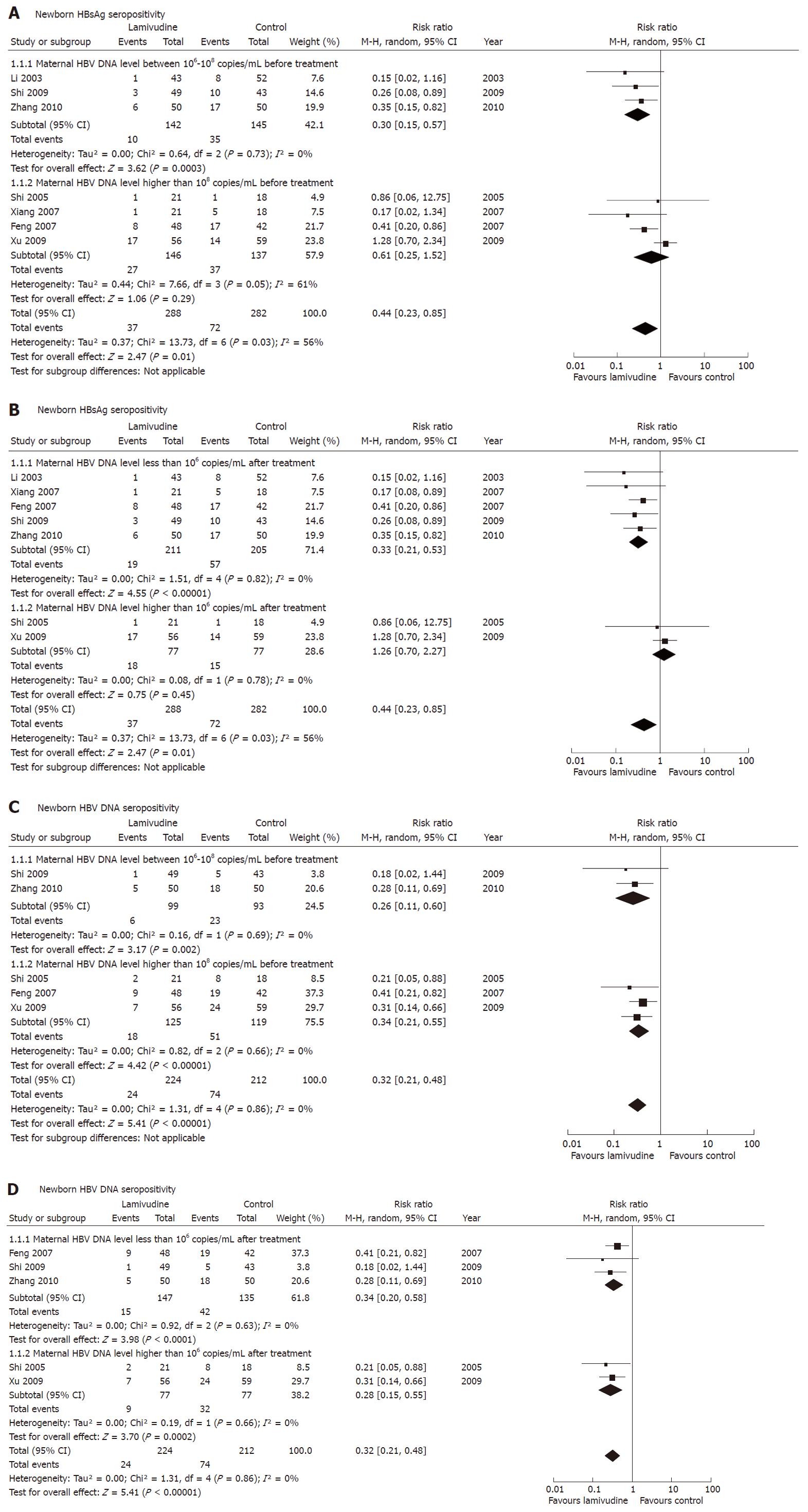
Figure 5 Influence of maternal viral load before or after lamivudine treatment on hepatitis B virus mother-to-child transmission as indicated by serum hepatitis B surface antigen or hepatitis B virus DNA of newborns within 24 h after birth.
Vertical line indicates no difference between compared treatments. Horizontal lines show 95% CIs. Squares indicate point estimates, and the size of the squares indicates the weight of each study in the meta-analysis. CI: Confidence interval; HBV: Hepatitis B virus; M-H random: Mantel-Haenszel random-effects model; RR: Risk ratio; HBsAg: Hepatitis B surface antigen.
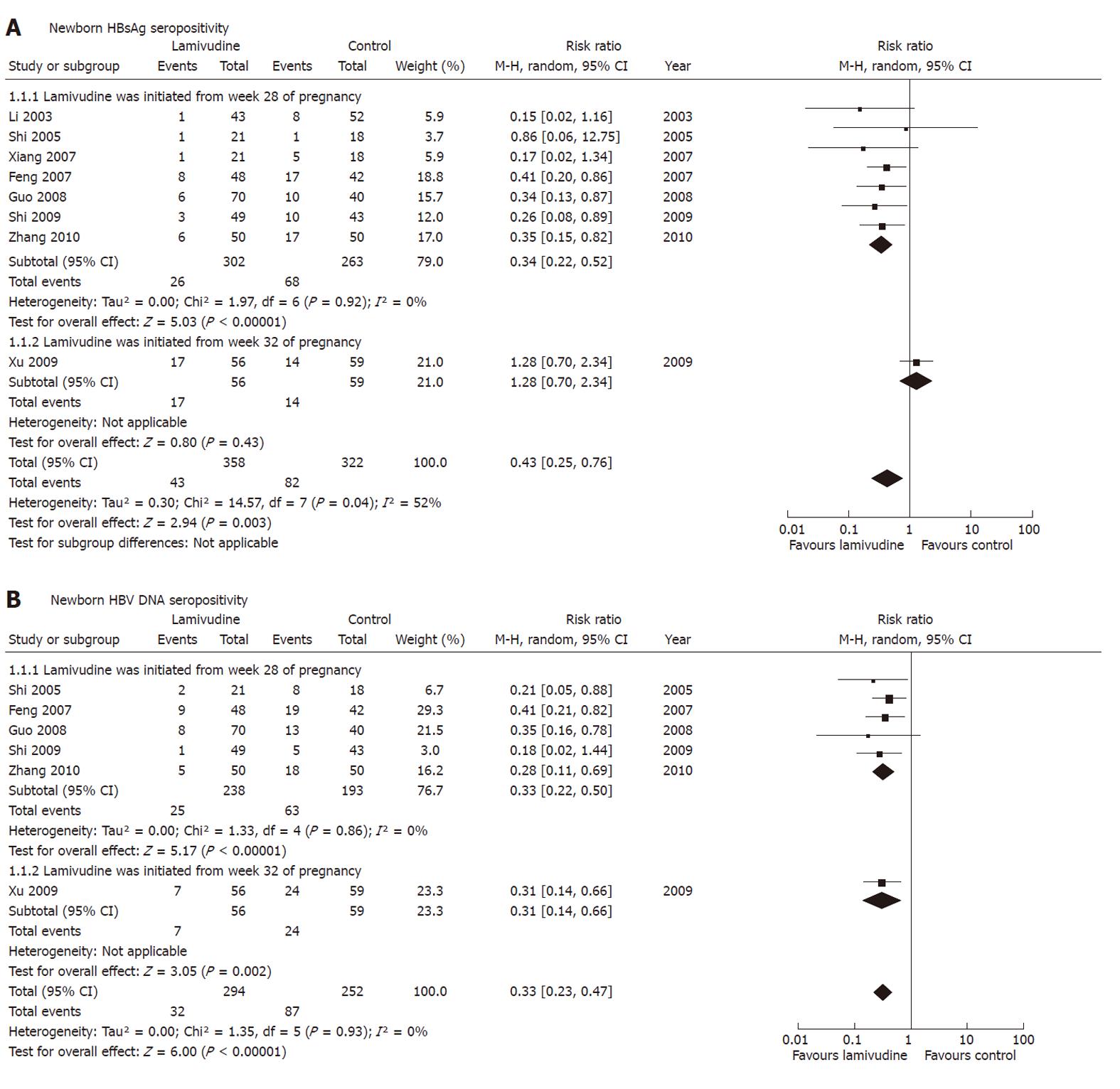
Figure 6 Effect of lamivudine treatment starting time on interruption of mother-to-child transmission indicated by newborn hepatitis B surface antigen or hepatitis B virus DNA.
Vertical line indicates no difference between compared treatments. Horizontal lines show 95% CIs. Squares indicate point estimates, and the size of the squares indicates the weight of each study in the meta-analysis. CI: Confidence interval; HBV: Hepatitis B virus; M-H random: Mantel-Haenszel random-effects model; RR: Risk ratio; HBsAg: Hepatitis B surface antigen.
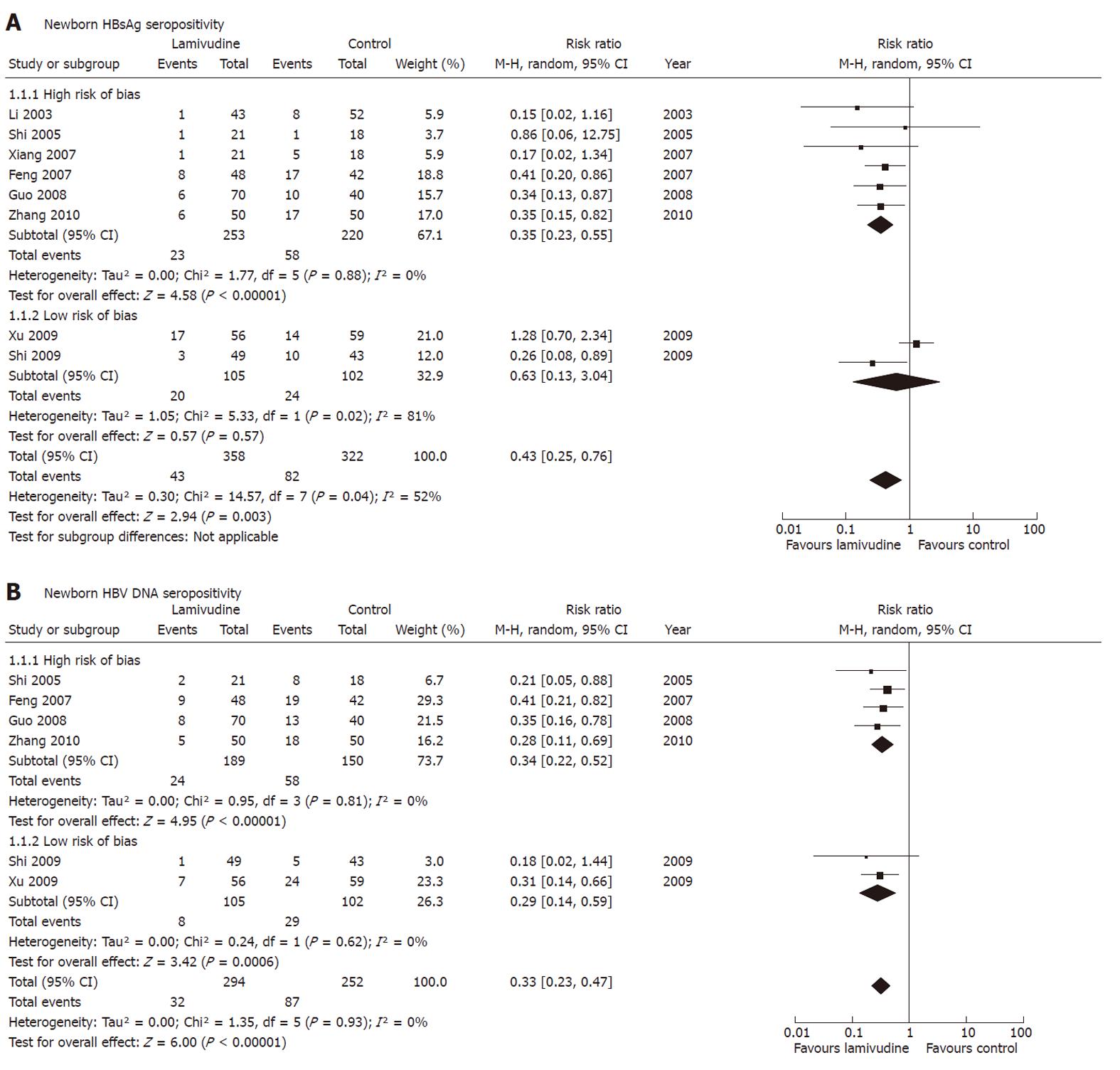
Figure 7 Efficacy of lamivudine treatment in “high-quality” studies or “low-quality” studies in interruption of mother-to-child transmission indicated by serum hepatitis B surface antigen or hepatitis B virus DNA of newborns.
Vertical line indicates no difference between compared treatments. Horizontal lines show 95% CIs. Squares indicate point estimates, and the size of the squares indicates the weight of each study in the meta-analysis. CI: Confidence interval; HBV: Hepatitis B virus; M-H random: Mantel-Haenszel random-effects model; RR: Risk ratio; HBsAg: Hepatitis B surface antigen.
- Citation: Han L, Zhang HW, Xie JX, Zhang Q, Wang HY, Cao GW. A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis B virus. World J Gastroenterol 2011; 17(38): 4321-4333
- URL: https://www.wjgnet.com/1007-9327/full/v17/i38/4321.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i38.4321