Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 14, 2011; 17(34): 3922-3932
Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3922
Published online Sep 14, 2011. doi: 10.3748/wjg.v17.i34.3922
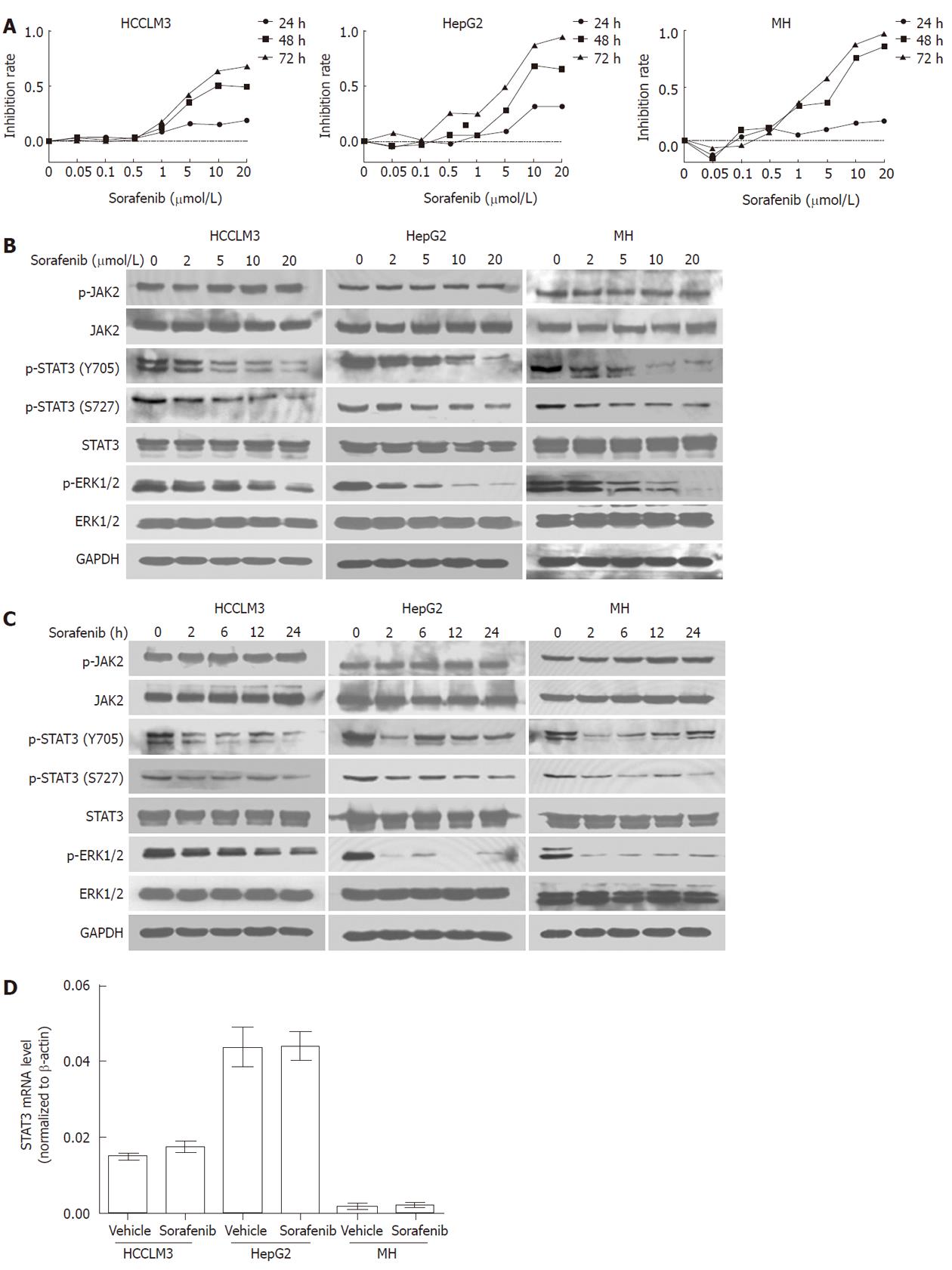
Figure 1 Inhibition of signal transducer and activator of transcription 3 signaling by sorafenib is associated with reduced cell proliferation.
A: Sorafenib inhibited cell proliferation in a time and dose-dependent manner, as assessed by the MTT assay; B: Sorafenib inhibited phosphorylation of signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase 1/2, but not janus kinase 2 (JAK2), in a dose-dependent manner. Hepatocellular carcinoma (HCC) cells were exposed to sorafenib for 2 h, and proteins were analyzed by Western blot; C: Sorafenib durably inhibited phosphorylation of STAT3 and ERK1/2 but not JAK2. HCC cells were treated with 10 μmol/L sorafenib for different durations, and cell lysates were analyzed by Western blotting; D: Sorafenib did not affect STAT3 mRNA levels in HCC cell lines (P > 0.05 for all). After 24-h sorafenib treatment (10 μmol/L), STAT3 mRNA levels were analyzed by qRT-PCR.
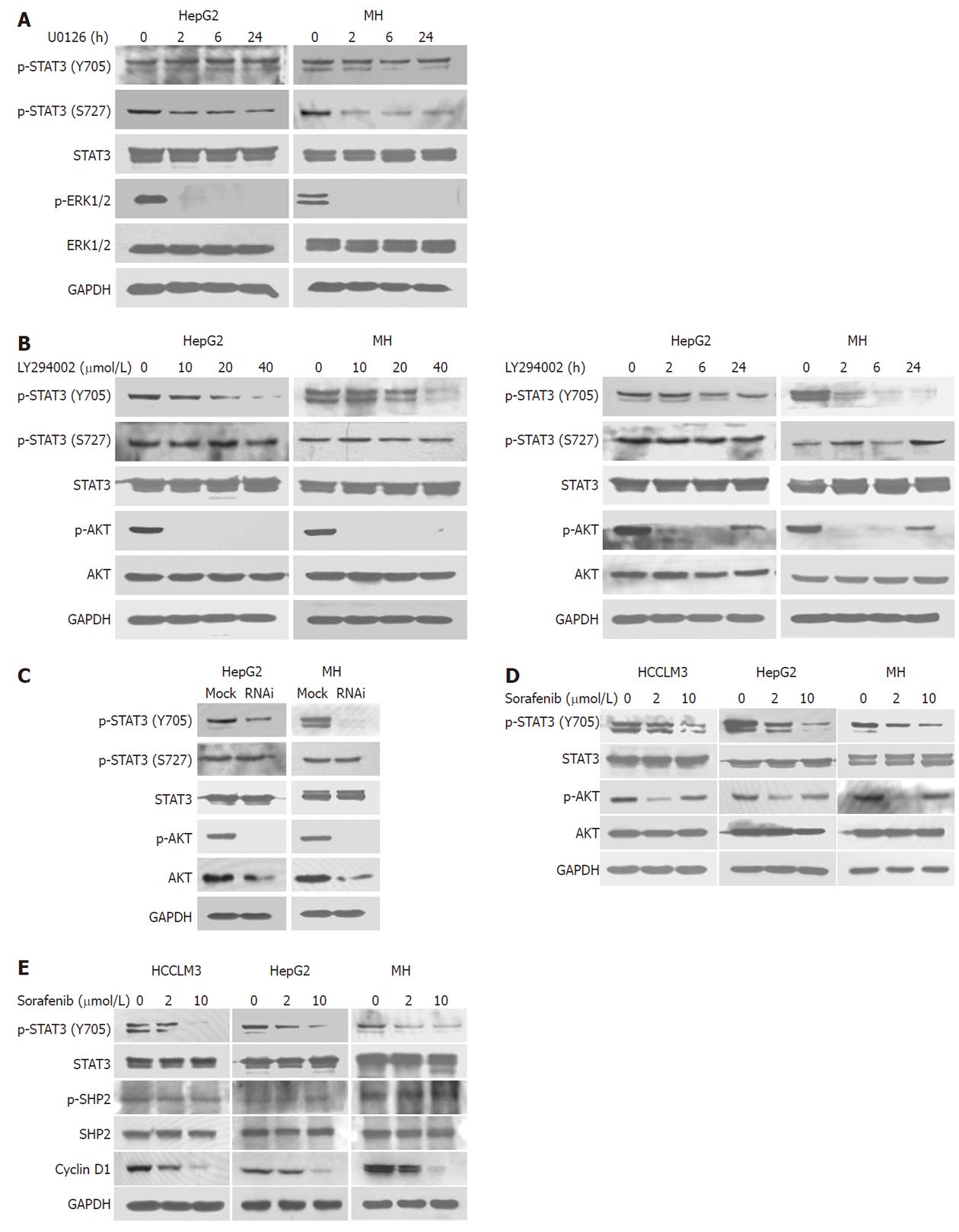
Figure 2 Inhibition of signal transducer and activator of transcription 3 signaling in hepatocellular carcinoma.
A: U0126 (20 μmol/L) durably inhibited phosphorylation of signal transducer and activator of transcription 3 (STAT3) (S727) in HepG2 and Morris hepatoma (MH) cell lines; B: A 2-h exposure to LY294002 inhibited phosphorylation of STAT3 (Y705) in HepG2 and MH cells in a dose-dependent manner (left). LY294002 (20 μmol/L) durably inhibited phosphorylation of STAT3 (Y705) in HepG2 and MH cells (right); C: Akt silencing by siRNA inhibited phosphorylation of STAT3 (Y705, but not S727) in HepG2 and MH cells; D: A 2-h exposure to sorafenib inhibited phosphorylation of Akt, mainly at low concentration (2 μmol/L) in HCCLM3, HepG2, and MH cells; E: Sorafenib promoted Y705 dephosphorylation and obviously reduced the expression levels of cyclin D1 regardless of shatterproof 2 (SHP2).
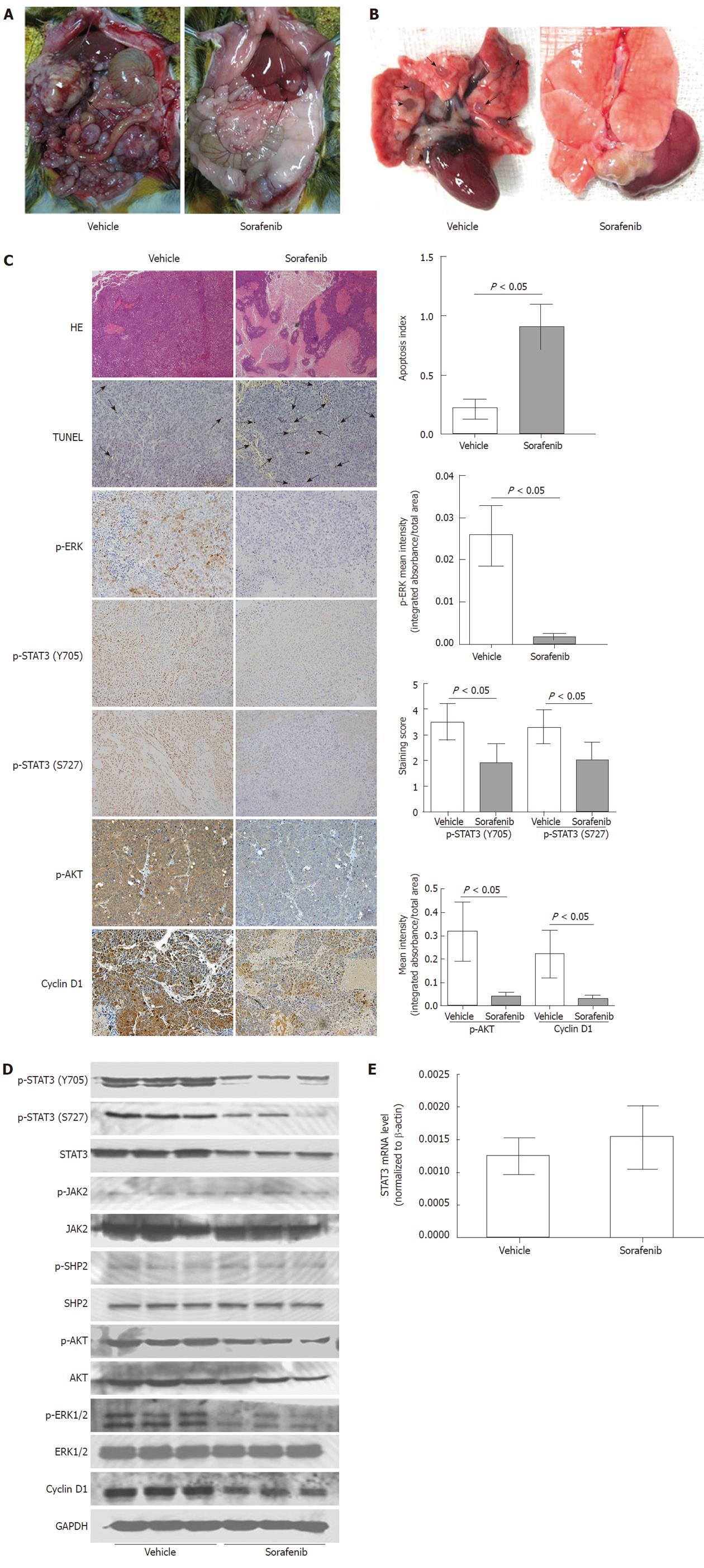
Figure 3 Inhibition of tumor growth and metastasis by sorafenib in vivo.
Rats with morris hepatoma (MH) tumors were treated with vehicle or sorafenib (30 mg/kg per day) starting on day 17 after tumor implantation. Tumors were removed on day 38 following implantation; A: Tumor growth and abdominal lymph node metastasis were reduced by sorafenib treatment (arrows); B: Sorafenib treatment reduced lung metastasis (arrows); C: Significant tumor necrosis in the sorafenib-treated group was visualized by hematoxylin-eosin staining (magnification, × 40). As shown by TUNEL (arrows), sorafenib also induced tumor cell apoptosis significantly (apoptosis index, 0.217 ± 0.825 vs 0.909 ± 0.189; P < 0.05; magnification, × 200). Immunohistochemical analysis showed that the expression levels of cyclin D1 and phosphorylation of signal transducer and activator of transcription 3 (STAT3) (Y705 and S727), Akt, and extracellular signal-regulated kinase (ERK) were significantly reduced by sorafenib treatment (P < 0.05 for all; magnification, × 200); D: Sorafenib reduced phosphorylation of STAT3, Akt, and ERK, but not Janus kinase 2 and shatterproof 2. Sorafenib also reduced the expression levels of cyclin D1 significantly; E: Sorafenib did not affect STAT3 mRNA levels in rat tumor tissues (P > 0.05).
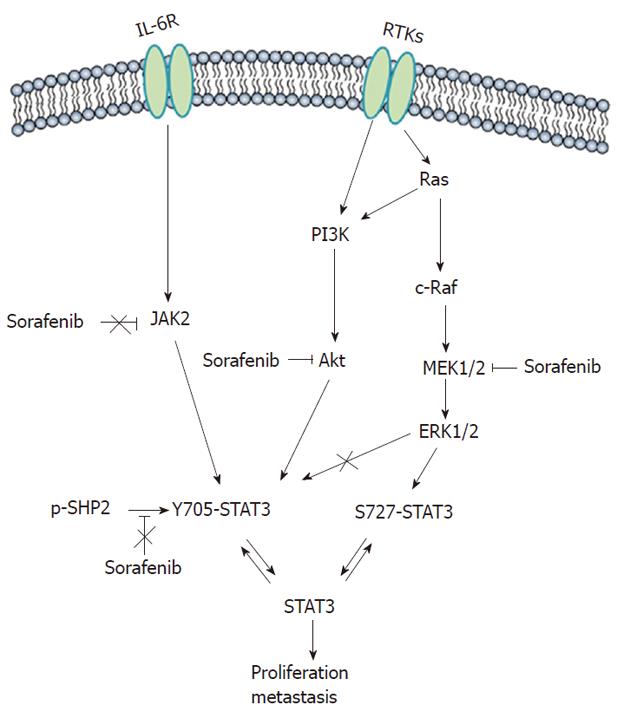
Figure 4 Signaling pathways involved in sorafenib-induced inhibition of signal transducer and activator of transcription 3 phosphorylation.
In hepatocellular carcinoma, extracellular signal-regulated kinase-related pathways regulate signal transducer and activator of transcription 3 (STAT3) phosphorylation at S727, and phosphatidylinositol-3-kinase (PI3K)/Akt can be responsible for phosphorylation at Y705. Thus, sorafenib inhibits STAT3 phosphorylation in part through blockade of the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)/STAT3 and PI3K/Akt/STAT3 signaling pathways, regardless of the Janus kinase 2 and phosphatase shatterproof 2.
- Citation: Gu FM, Li QL, Gao Q, Jiang JH, Huang XY, Pan JF, Fan J, Zhou J. Sorafenib inhibits growth and metastasis of hepatocellular carcinoma by blocking STAT3. World J Gastroenterol 2011; 17(34): 3922-3932
- URL: https://www.wjgnet.com/1007-9327/full/v17/i34/3922.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i34.3922