Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 21, 2011; 17(3): 273-282
Published online Jan 21, 2011. doi: 10.3748/wjg.v17.i3.273
Published online Jan 21, 2011. doi: 10.3748/wjg.v17.i3.273
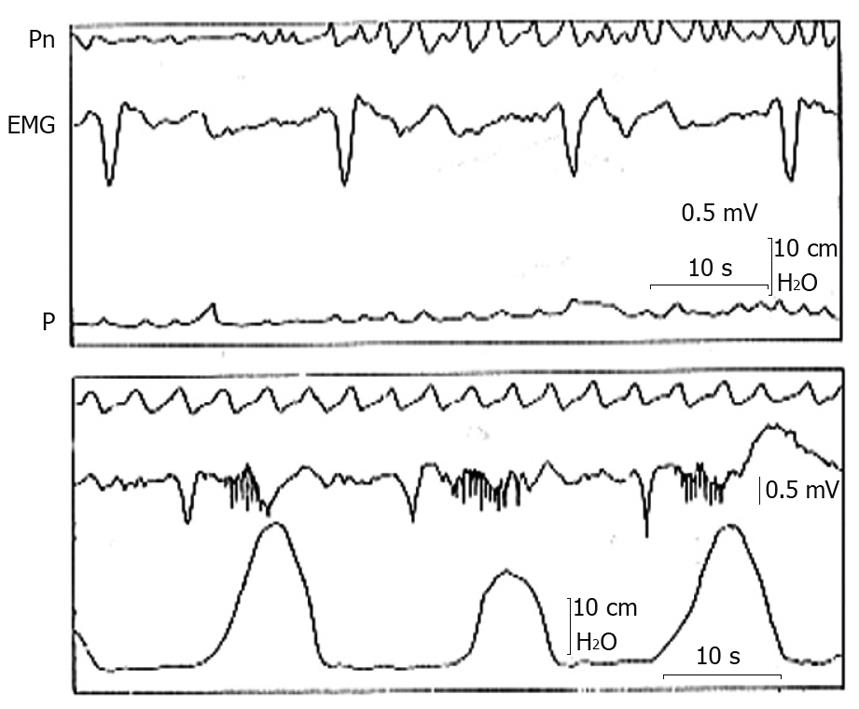
Figure 1 Gastric myoelectric and pressure activities recorded with an intraluminal electromyographic and manometric technique from the gastric antrum of a healthy subject during a period of absence of pressure waves (top tracing) and a period of contractile activity (bottom tracing).
Note the slow waves of normal morphology and the frequency of 3 cycles/min that in correspondence with the contractions are followed by bursts of spikes[16]. EMG: Gastric myoelectric; Pn: Pneumogram; P: Pressure.
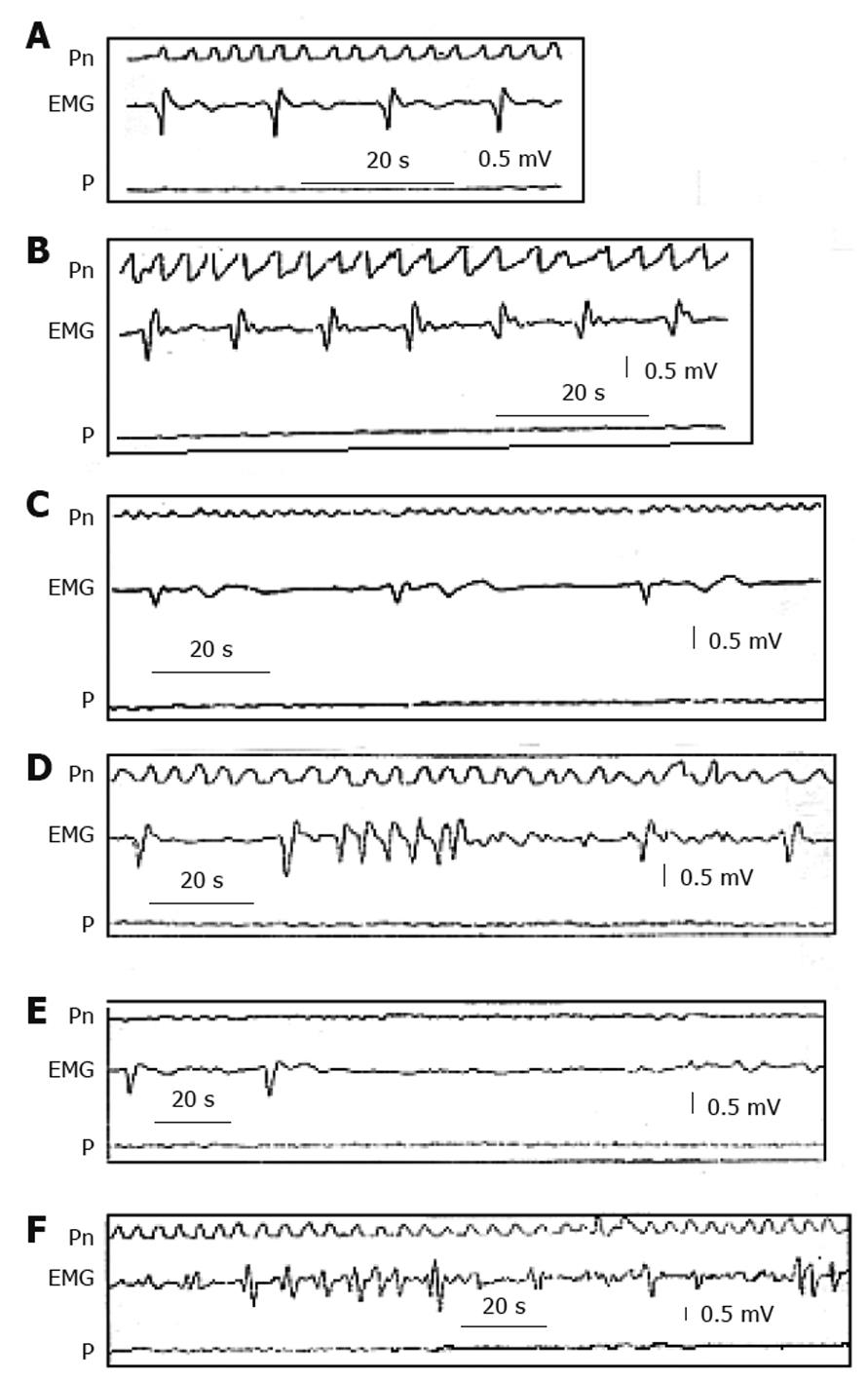
Figure 2 Electrogastrographic alterations.
Series of alterations in the gastric myoelectric activity recorded with an electromyographic and manometric technique from the gastric antrum of patients with severe gastroparesis in confront with (A) normal myoelectric activity recorded in a healthy subject showing slow waves of normal morphology and frequency of 3 cycles/min. B: Tachygastria; C: Bradygastria; D: Run of high frequency tachygastria; E: Bradyarrhythmia; F: Complete disorganization of myoelectric activity (“gastric fibrillation”). Note that all these alterations are associated with absence of gastric contractions[1]. EMG: Gastric myoelectric; Pn: Pneumogram; P: Pressure.
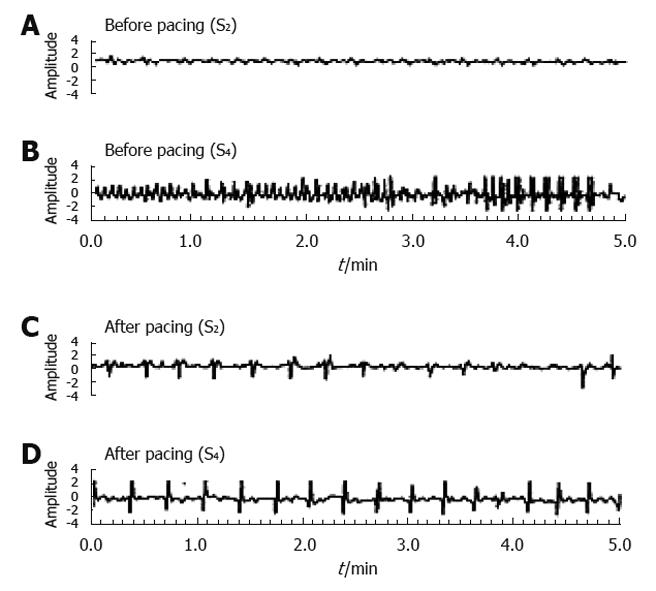
Figure 3 Normalization of ectopic tachygastria with low-frequency/high-energy gastric electrical stimulation [pacing frequency: 3.
2 cycles/min (cpm), pulse width: 300 ms, amplitude: 4 mA] in a patient with gastroparesis. The pacing was carried out in the proximal corpus and the recording electrodes were in the mid gastric corpus (S2) and in the gastric antrum (S4). Before pacing a slow wave of about 3.5 cpm was recorded at S2 (A) and tachyarrythmias at S4 (B), whereas the pacing with a frequency of 3.2 cpm entrained gastric slow waves at S2 (C) and S4 (D)[44].
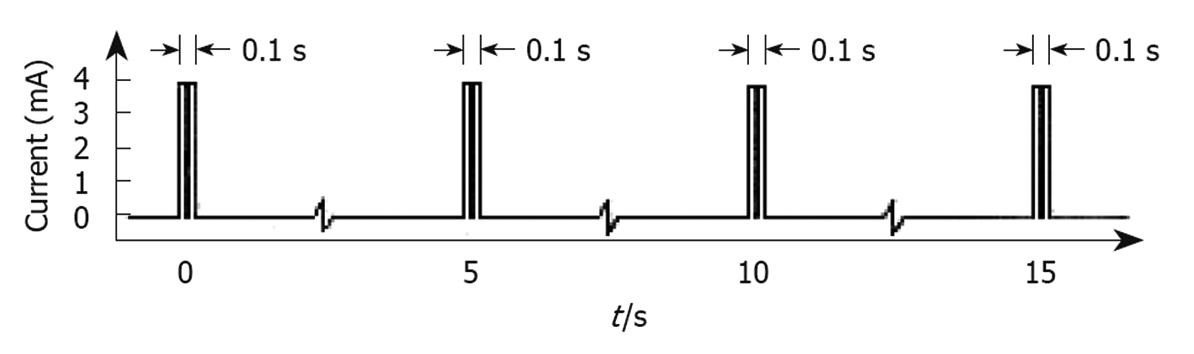
Figure 4 Type of electrical stimulation used by the Enterra system.
Short bursts of short duration rectangular pulses (330 μs each) with amplitude of 4 mA were given at a frequency of 14 Hz in each burst. Bursts in turn lasted 0.1 s and were delivered every 5 s[39].
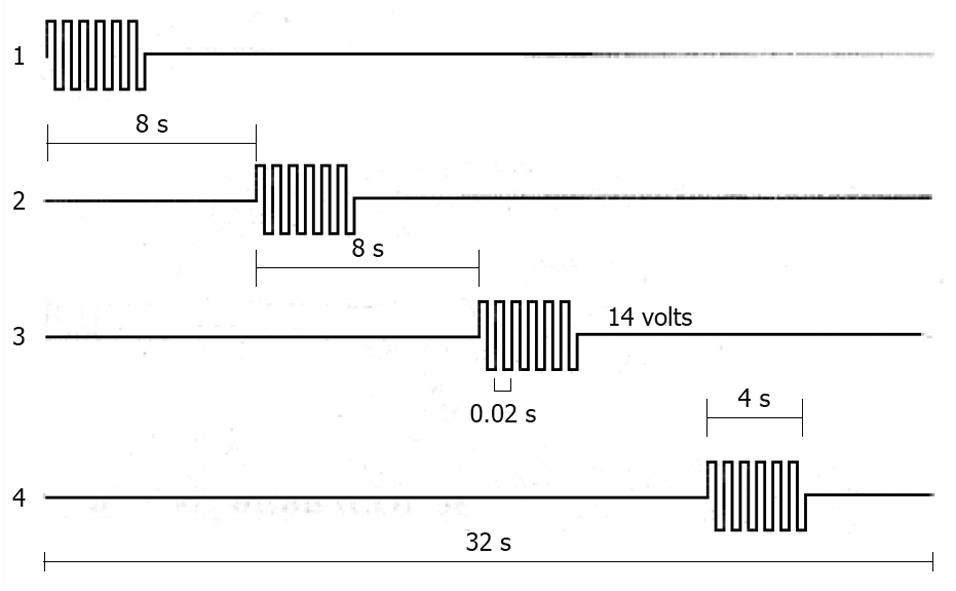
Figure 5 Characteristics of one sequential gastric pacing stimulation protocol in dogs are shown from the proximal (1) to the distal (4) electrodes, that were positioned along the gastric corpus and antrum at 4 cm interval.
Four second duration pulse trains with an amplitude of 14 V and a frequency of 50 Hz were delivered in synchronized fashion with a 4 s lag between adjacent stimulus sites[89].
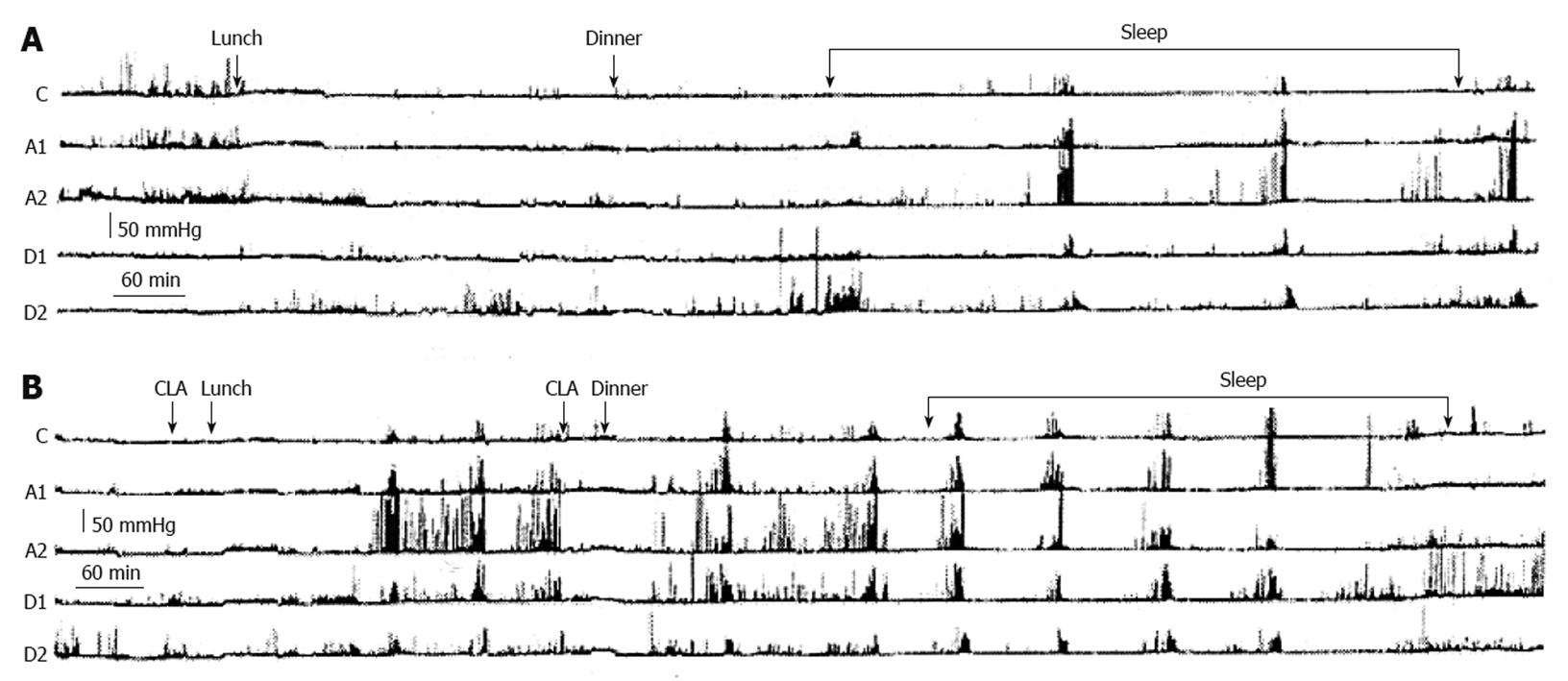
Figure 6 Two ambulatory 24-h gastroduodenal recordings (A and B) carried out on two separate days in a patient with apparently refractory gastroparesis by means of a probe with 5 miniaturized electronic pressure transducers, 5 cm apart: one in the corpus (C), two in the antrum (A1 and A2) and two in the duodenum (D1 and D2).
On the first day (A) the recording was carried out without drug administration and on the second day (B) with clarithromycin (CLA) administration. A: On the first day, the postprandial gastric motor activity was very low and only three activity fronts of the Migrating Motor Complex were observed, two during the night and one early in the morning; B: On the second day, the oral administration of clarithromycin 30 min before lunch was followed about 3 h later by a burst of powerful peristaltic contractions starting in the stomach and progressing in the duodenum, followed by two others bursts at about 80 min intervals. The oral administration of clarithromycin 30 min before dinner induced after about 2.4 h a series of six bursts of powerful peristaltic waves in the stomach and duodenum at 80-100 min intervals[103].
- Citation: Bortolotti M. Gastric electrical stimulation for gastroparesis: A goal greatly pursued, but not yet attained. World J Gastroenterol 2011; 17(3): 273-282
- URL: https://www.wjgnet.com/1007-9327/full/v17/i3/273.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i3.273