Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Jun 7, 2011; 17(21): 2674-2680
Published online Jun 7, 2011. doi: 10.3748/wjg.v17.i21.2674
Published online Jun 7, 2011. doi: 10.3748/wjg.v17.i21.2674
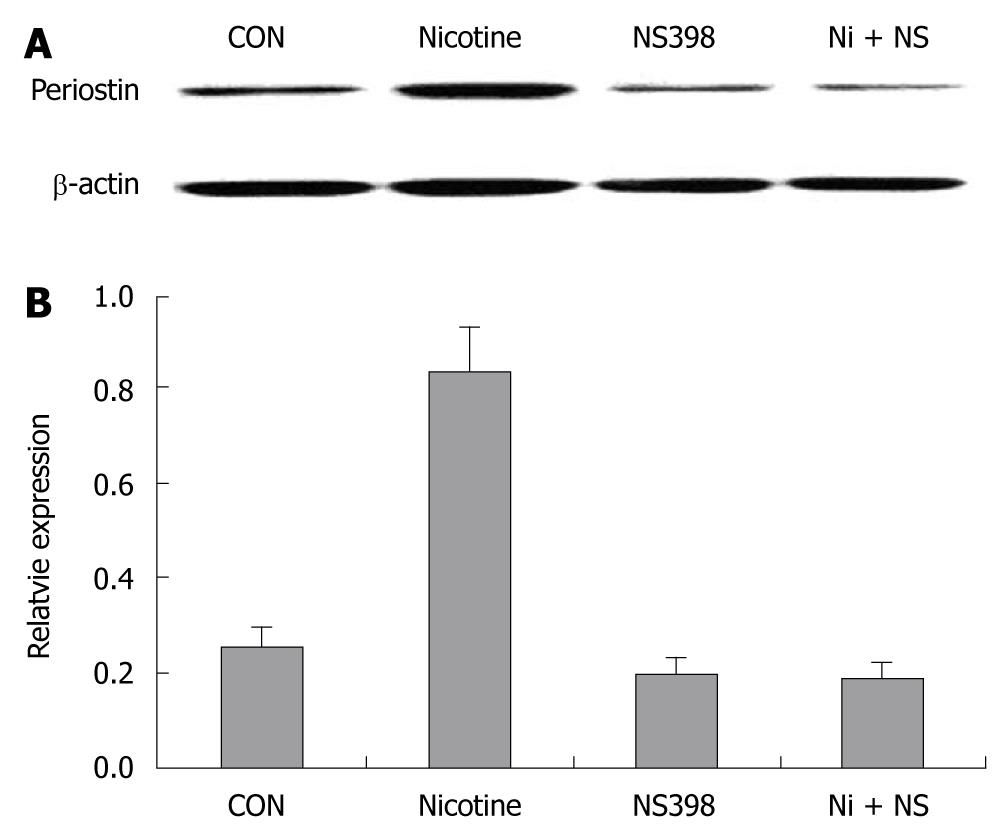
Figure 1 COX-2 inhibitor NS398 showed inhibitory effects on nicotine (200 ng/mL)-induced periostin expression after 24 h of treatment.
COX-2-specific inhibitor NS398 (10 μmol/L) was applied to SGC-7901 cells 6 h before nicotine (200 μg/mL) treatment. A: Western blotting bands of periostin protein in four groups of gastric cancer cells; B: Quantification analysis demonstrated significantly higher relative expression of periostin in the nicotine group than in the other three groups (P < 0.05). Notes: CON: Control; Nicotine: Treated with nicotine; NS398: Treated with COX-2-specific inhibitor NS398; Ni + NS: Combined treatment with nicotine and NS398.
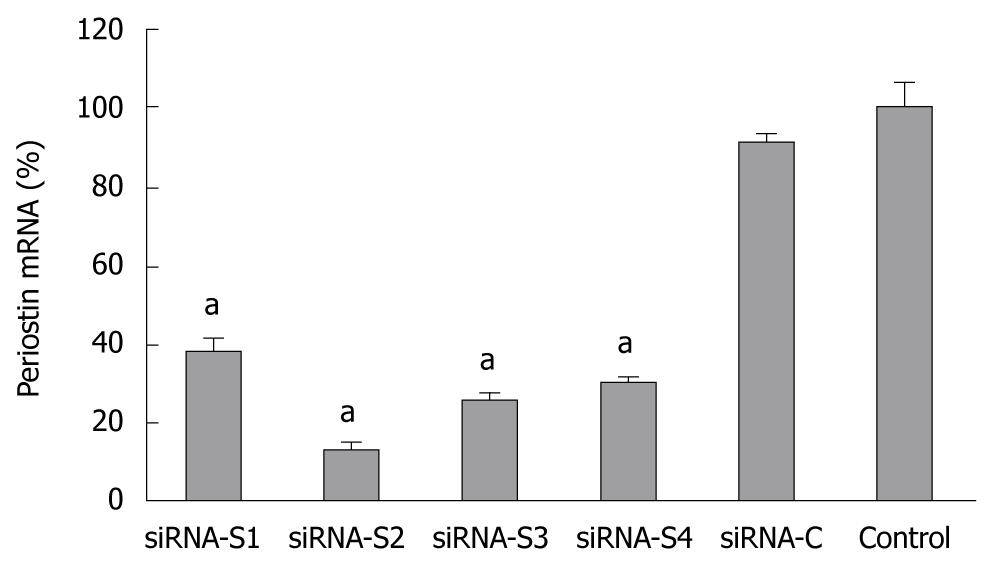
Figure 2 Relative expression of periostin mRNA 48 h after transfection by siRNA plasmid.
The silencing effect of the transfection of periostin siRNA plasmid in SGC-7901 cells was confirmed by quantitative reverse transcription-polymerase chain reaction 48 h after transfection. Four representative results are shown (S1-S4). SiRNA-C indicates gastric cancer cells with control plasmid. Control indicates gastric cancer cells without transfection. aP < 0.05 vs control. The Y ordinate indicates the ratio of periostin mRNA normalized to that of house-keeping genes. Notes: siRNA-S1, siRNA-S2, siRNA-S3, siRNA-S4: four representative results of periostin siRNA transfection; siRNA-C: vacant siRNA-control; control: SGC-7901 cells without transfection.
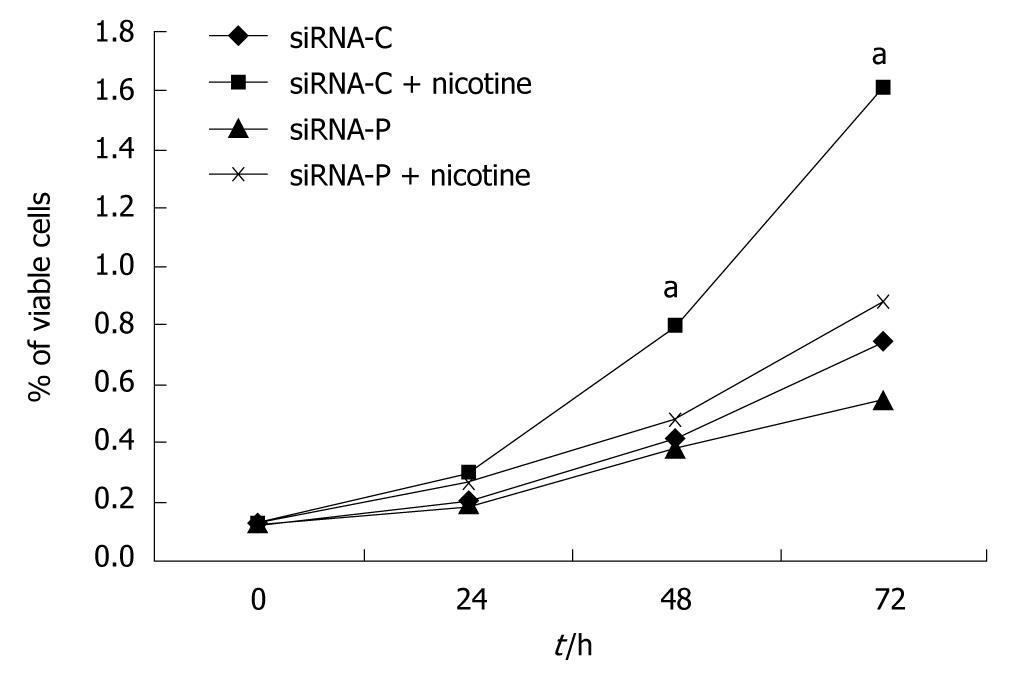
Figure 3 Periostin siRNA inhibited nicotine-promoted cell growth in gastric cancer cells.
SGC-7901 cells with stable expression of siRNA-periostin (siRNA-P) and vacant siRNA-control (siRNA-C) were treated with nicotine. Cells were then lysed to analyze cell viability using the methylthiazolyldiphenyl-tetrazolium bromide assay at 0, 24, 48, and 72 h of treatment, and growth curves were constructed. The viability of the siRNA-C+nicotine group was significantly higher than those of the other three groups at the 48 and 72 h time points (P < 0.05), which is indicated by “a”. Notes: siRNA-C: vacant siRNA-control; siRNA-P transfected with periostin.
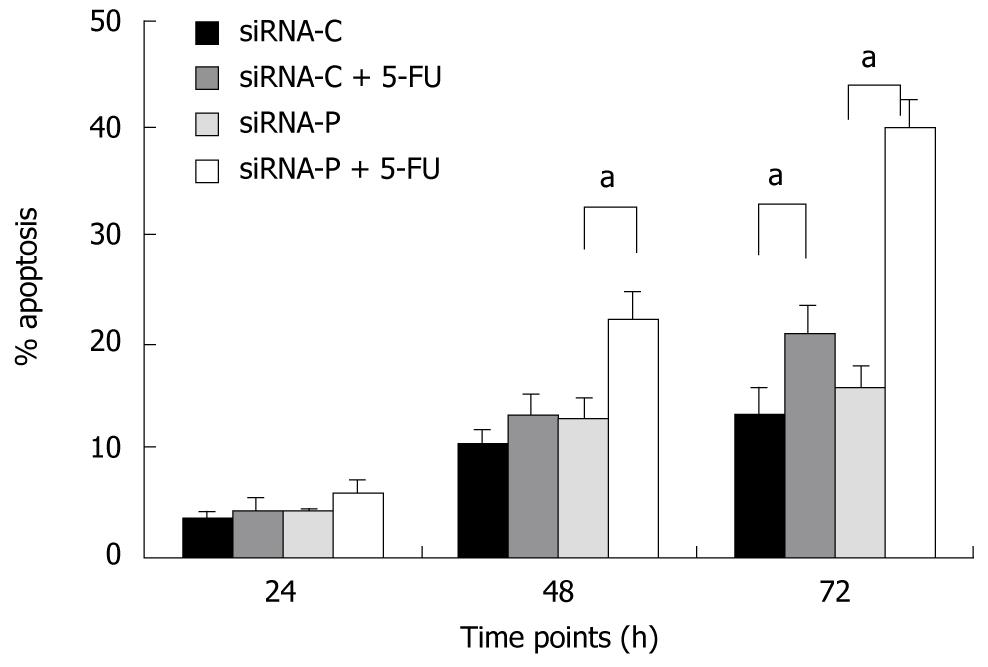
Figure 4 Periostin siRNA increased the sensitivity of gastric cancer cells to 5-fluorouracil-induced apoptosis.
SGC-7901 cells with stable expression of siRNA-periostin (siRNA-P) and vacant siRNA-control (siRNA-C) were treated or not with chemotherapy agent 5-FU. siRNA-periostin significantly increased the sensitivity of 5-FU treated gastric cancer cells both at 48 h and 72 h (aP < 0.05). Notes: siRNA-C: Vacant siRNA-control; siRNA-P: Transfected with periostin; 5-FU: 5-fluorouracil (50 mg/L).
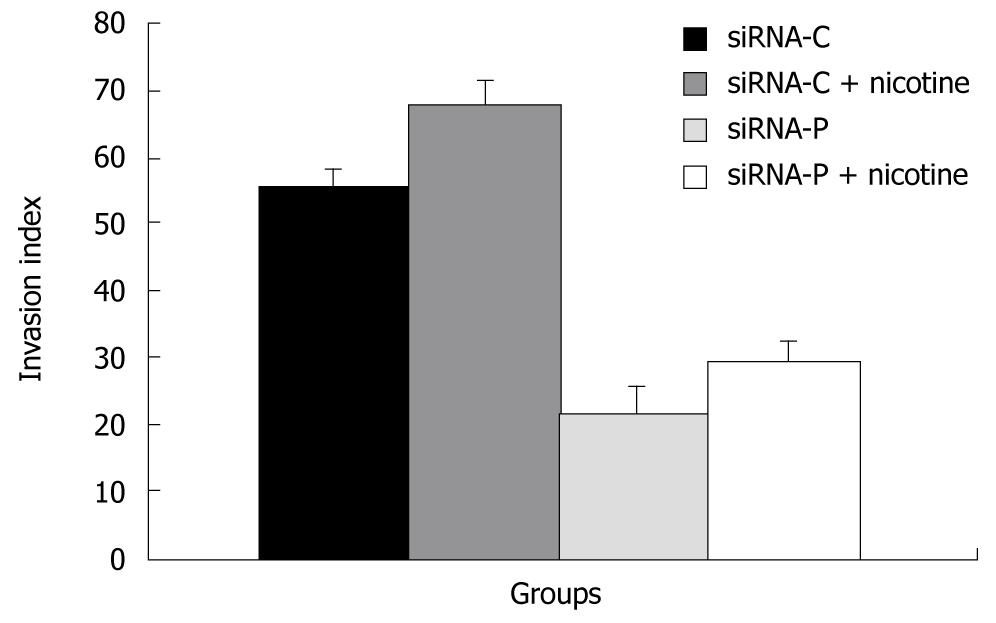
Figure 5 The effect of periostin siRNA on the ability of gastric cancer cells to invade.
SGC-7901 cells with stable expression of siRNA-periostin (siRNA-P) and vacant siRNA-control (siRNA-C) were treated with nicotine and evaluated for cell invasion using the Boyden chamber invasion assay.
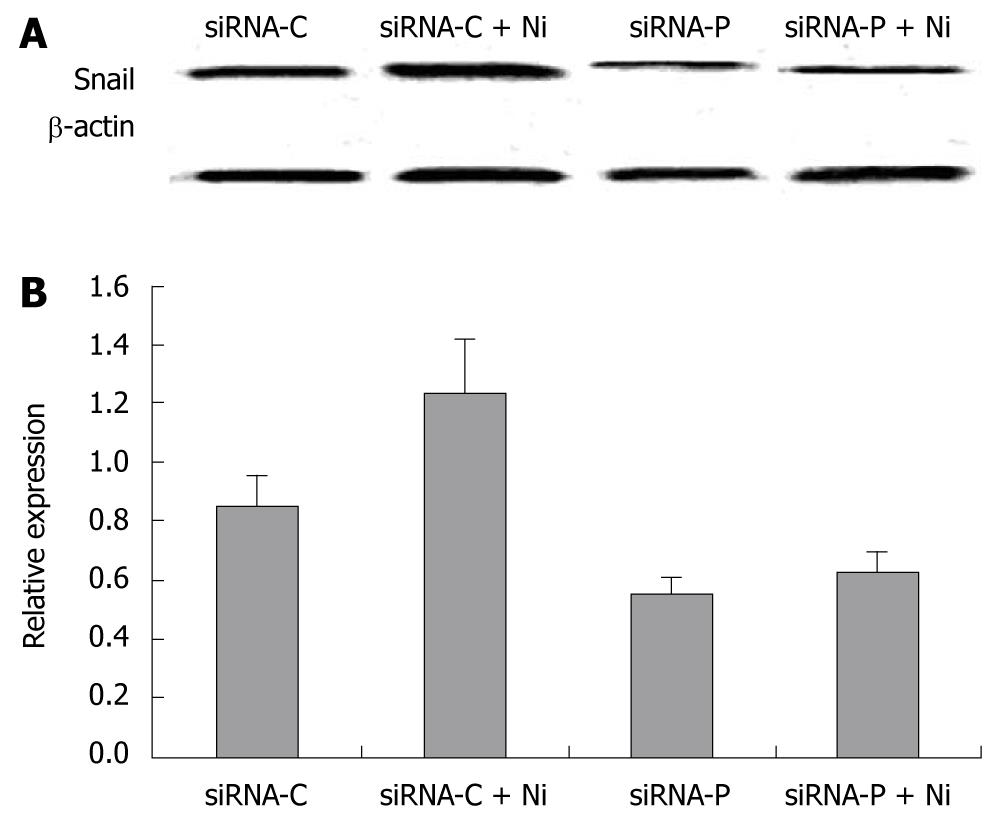
Figure 6 The inhibitory effect of periostin siRNA on nicotine-induced Snail protein expression.
A: Western blotting bands of Snail protein in four groups of gastric cancer cells. B: Relative expression of Snail in the above four groups. Nicotine significantly increased the relative expression of Snail protein in gastric cancer cells (P < 0.05). However, there were no significant differences in Snail expression between the siRNA-P group and the siRNA-P+nicotine group (P > 0.05). Notes: siRNA-C: vacant siRNA-control; siRNA-P: Transfected with periostin; Ni: Treated by 200 ng/mL nicotine.
- Citation: Liu Y, Liu BA. Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J Gastroenterol 2011; 17(21): 2674-2680
- URL: https://www.wjgnet.com/1007-9327/full/v17/i21/2674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i21.2674