Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. May 28, 2011; 17(20): 2482-2499
Published online May 28, 2011. doi: 10.3748/wjg.v17.i20.2482
Published online May 28, 2011. doi: 10.3748/wjg.v17.i20.2482
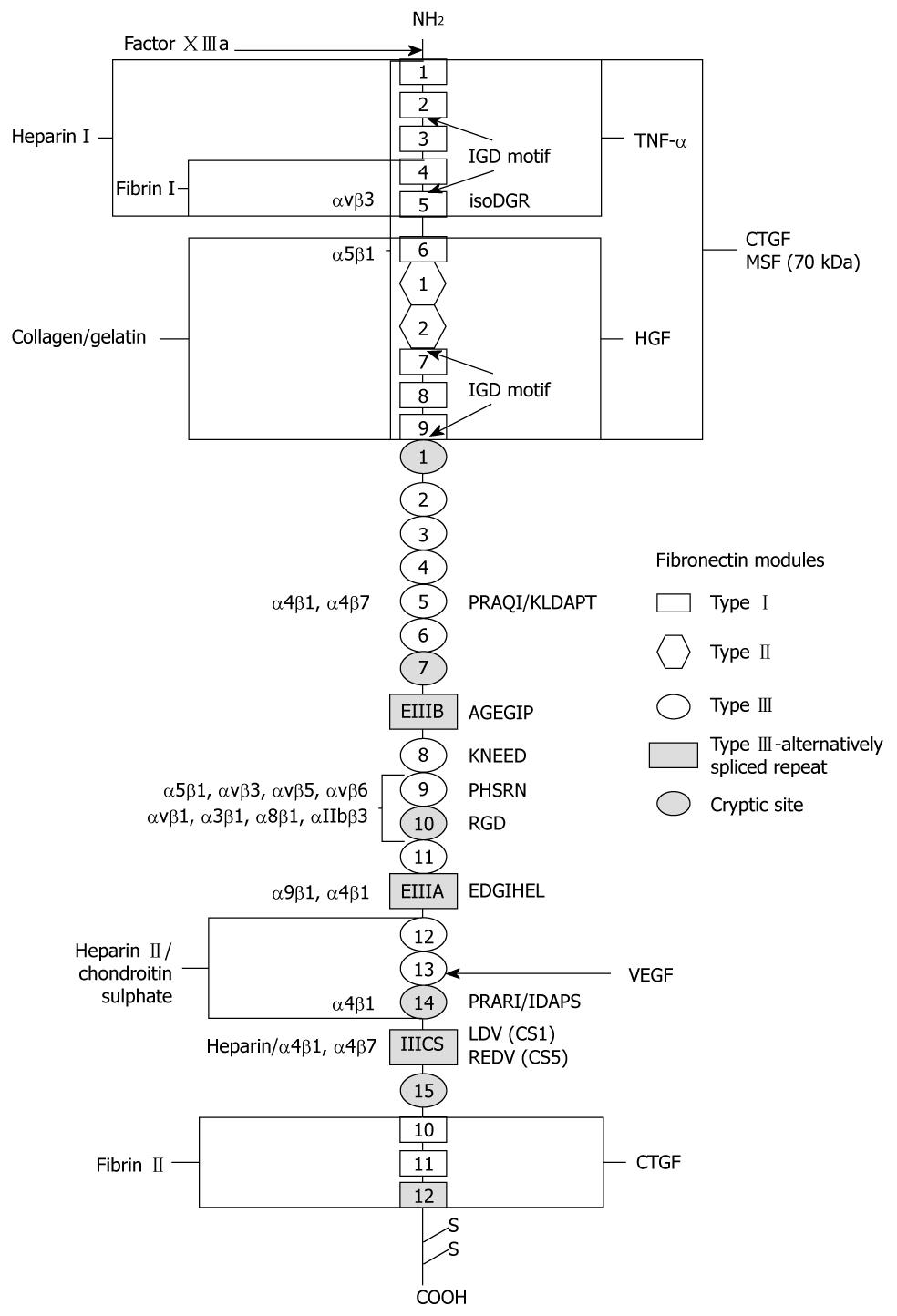
Figure 1 Domain structure and interaction sites of fibronectin.
Fibronectin is a dimer comprised of two subunits which are covalently joined by two disulfide bridges near the COOH-terminus. Each subunit consists of three types of homologous structural domains called I, II, and III. Recognition sequences, integrin binding sites, cryptic sites and interactive regions of the molecule are labeled.
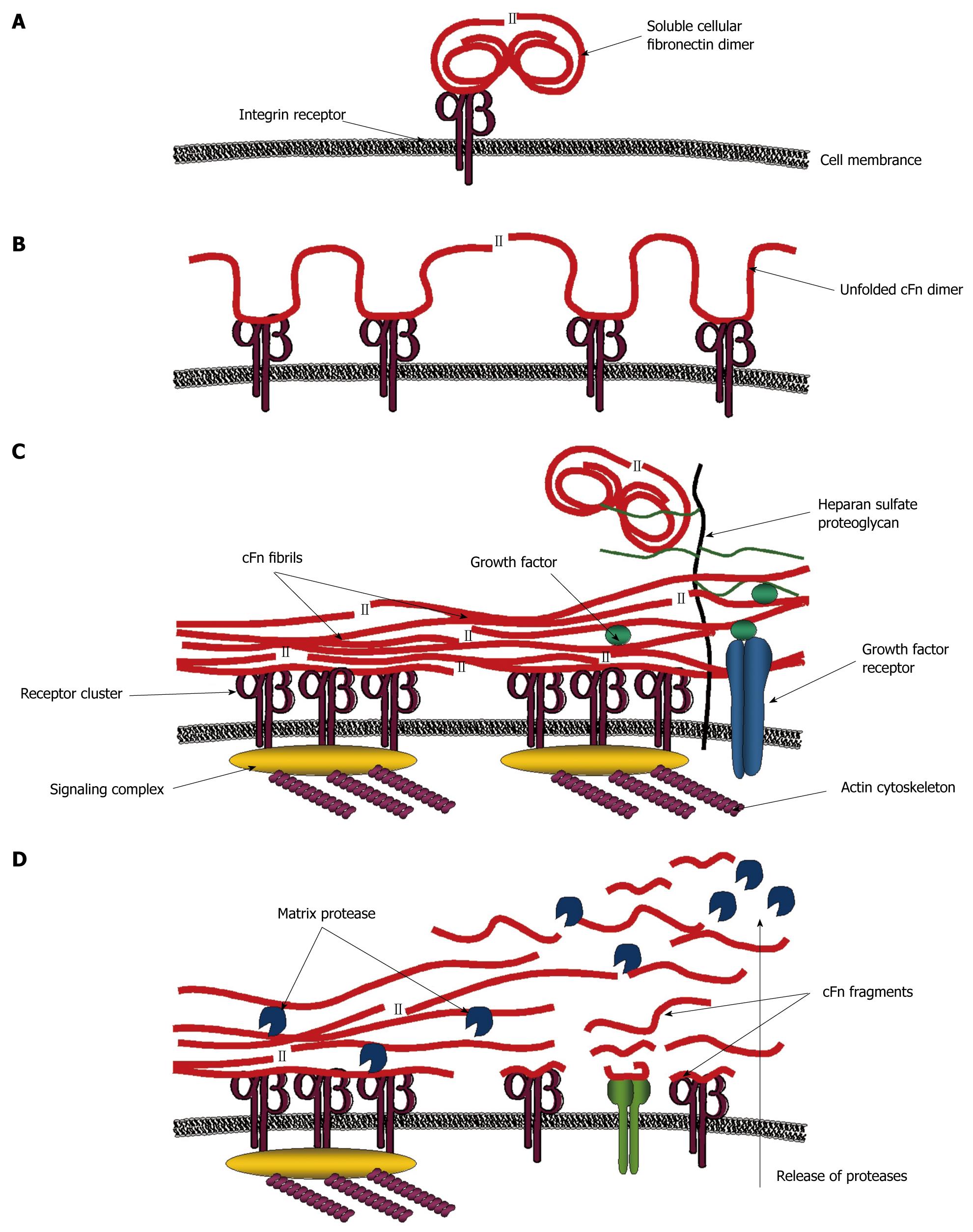
Figure 2 Model for signaling processes mediated by fibronectin.
A: Initial signals are mediated when soluble compact cellular fibronectin (cFn) binds a cognate receptor; B: This causes cFn to unfold and interact with other receptors inducing further signals; C: Receptor clustering and the formation of signaling complexes lead to the reorganization of the actin cytoskeleton which creates tensile forces, conveyed through the integrin receptors to further stretch cFn into fibrillar form. Exposed cryptic sites interact with other cFn fibrils in matrix assembly. Access to growth factors and other molecules is regulated by cFn binding. Heparan sulfate proteoglycans also bind to cFn and recruit distant molecules closer to the cell surface; D: All of these interactions create a cascade of different signals, some of which promote matrix protease release. Resulting cFn fragments activate additional intracellular signaling pathways. Thus, cFn can regulate cell behavior via numerous different mechanisms.
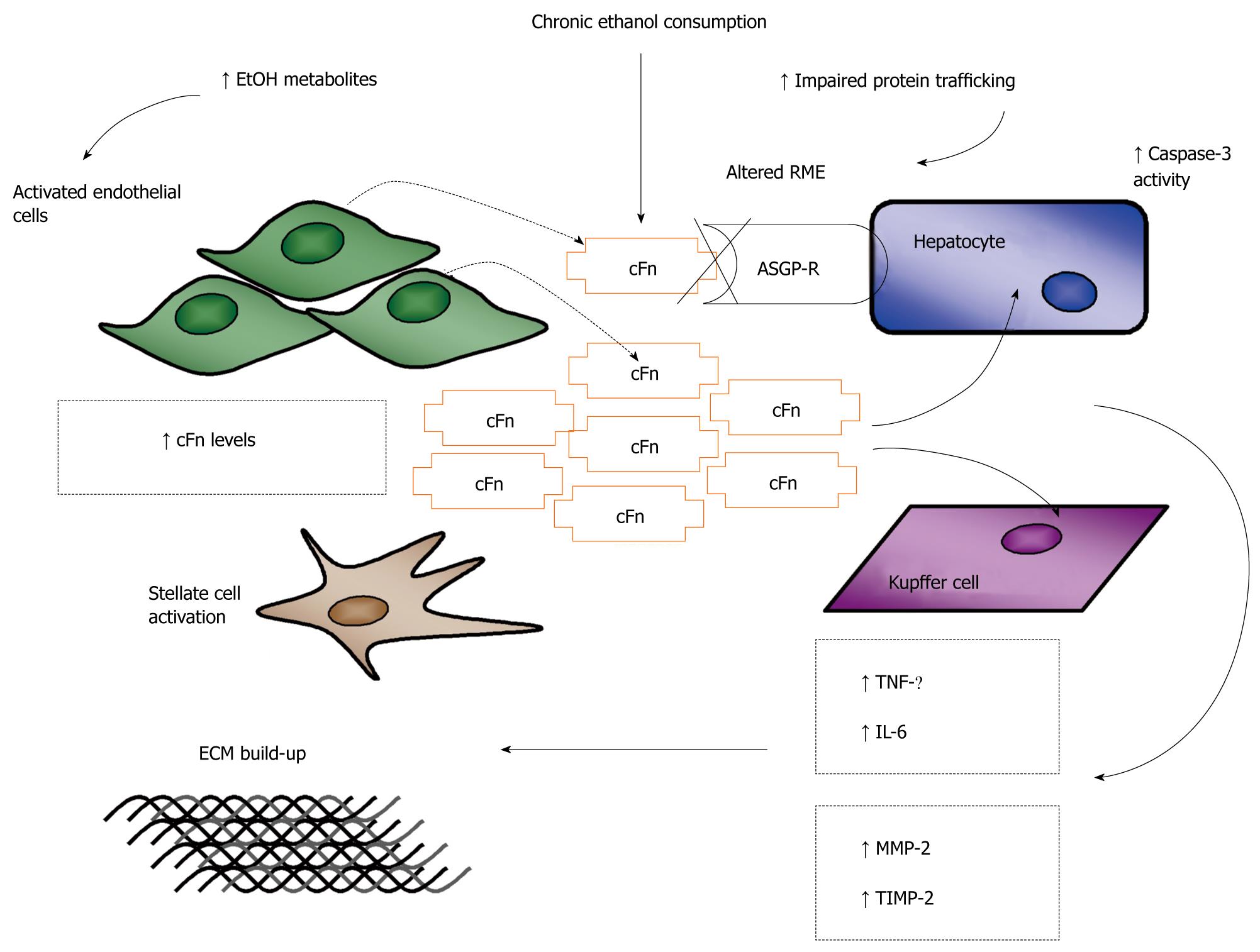
Figure 3 Schematic representation of the proposed model of ethanol-induced liver injury linking altered asialoglycoprotein receptor clearance of cellular fibronectin with hepatocyte and kupffer cell activation by the accumulating protein.
The alcohol induced up regulation of cellular fibronectin (cFn) production by sinusoidal endothelial cells (SECs) and its impaired clearance by the hepatocyte-specific asialoglycoprotein receptor (ASGP-R) leads to the accumulation of cFn in the liver. Hepatocytes (HCs) and kupffer cells (KCs) are stimulated by cFn to produce the pro-inflammatory/pro-fibrogenic cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-6, which further activate hepatic stellate cell (HSCs) stimulating their transformation to the pro-fibrogenic phenotype. HCs and KCs are also stimulated to produce the matrix degrading enzyme, matrix metalloproteinase (MMP)-2 and its corresponding inhibitor, tissue inhibitor of metalloproteinase (TIMP)-2. Greater levels of TIMP-2 are secreted resulting in the inhibition of MMP-2 activity and subsequent build-up of the extracellular matrix (ECM), characteristic of the early onset of fibrotic liver damage. RME: Receptor mediated endocytosis.
- Citation: Aziz-Seible RS, Casey CA. Fibronectin: Functional character and role in alcoholic liver disease. World J Gastroenterol 2011; 17(20): 2482-2499
- URL: https://www.wjgnet.com/1007-9327/full/v17/i20/2482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i20.2482