Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 21, 2011; 17(11): 1383-1399
Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1383
Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1383
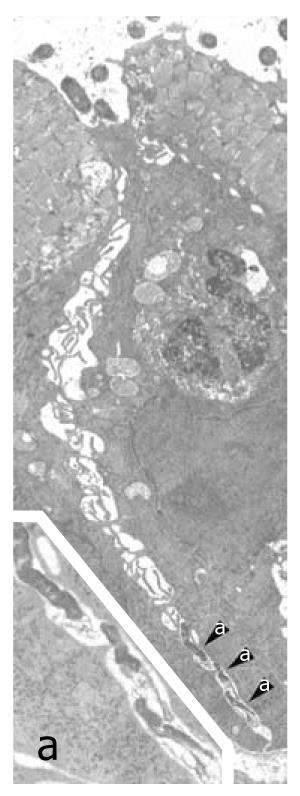
Figure 1 Helicobacter pylori penetration in human gastric epithelium in vivo.
Three Helicobacter pylori (H. pylori) organisms (enlarged in a; 16 800 ×) lying in the deep intercellular intraepithelial space, just above the basal membrane. Note also luminal bacteria (top) overlying an apparently preserved tight junction, dilation of the underlying intercellular space, filled with lateral membrane plications, and an intraepithelial granulocyte (middle right, 6300 ×). Reprinted from Necchi et al[57], with permission from Elsevier.
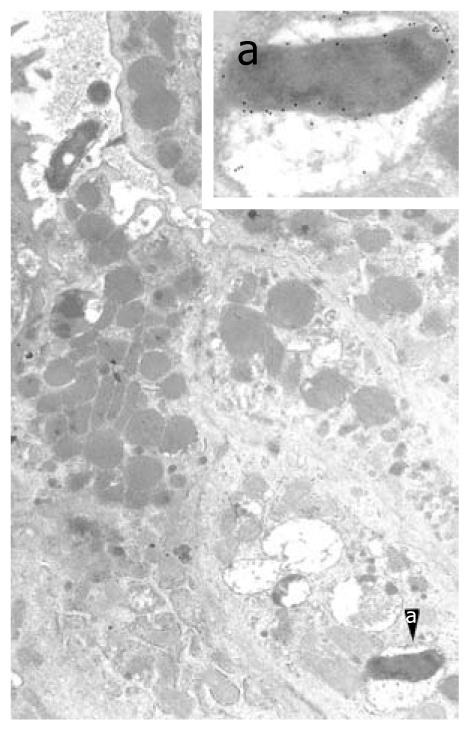
Figure 2 Intracellular Helicobacter pylori in human gastric epithelium in vivo.
A well-preserved Helicobacter pylori (H. pylori) organism in a cytoplasmic vacuole, enlarged in a (55 200 ×; 15 nm gold particles) to show immunoreactivity with anti-VacA antibody. Note two H. pylori in a luminal cleft (top left, 13 500 ×). Reprinted from Necchi et al[57], with permission from Elsevier.
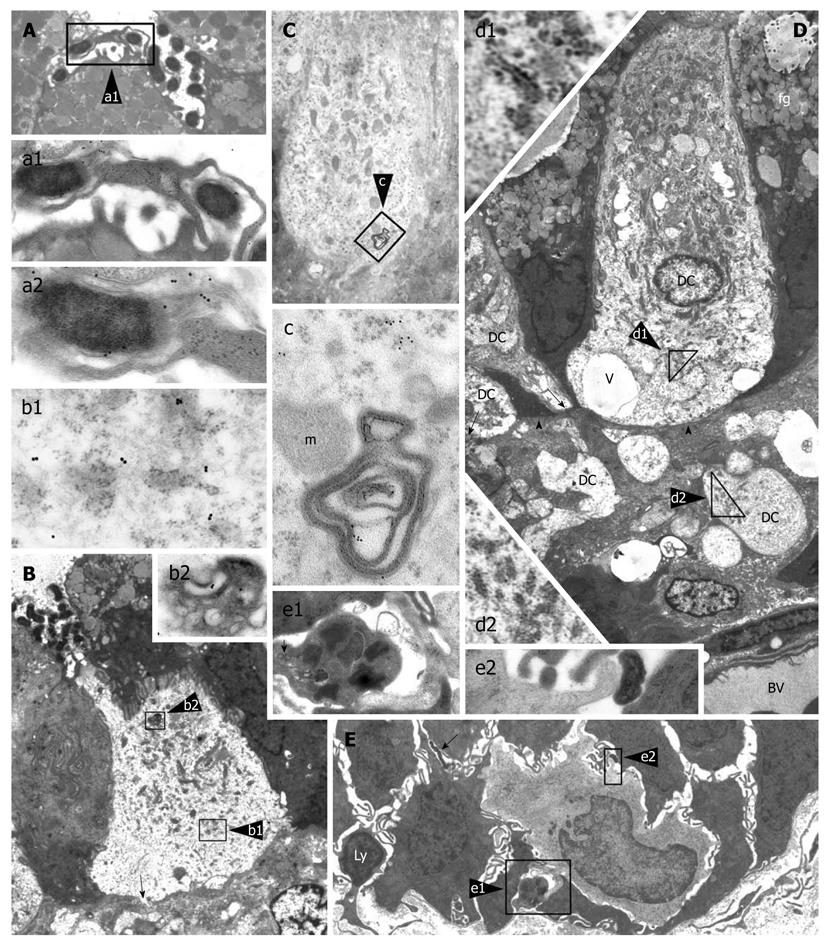
Figure 3 Intraepithelial dendritic cells in Helicobacter pylori-positive human gastric biopsies with active inflammation.
A (5000 ×): Luminal ending of a dendritic cell (DC) process abutting on a collection of Helicobacter pylori (H. pylori); enlarged in a1 (16 000 ×) to show envelopment of a bacterium by caliceal veils (right); note in a2 (28 000 ×; detail of a1), close adherence of a clubbed process (bottom left) to another bacterium showing VacA immunoreactivity of outer membrane and flagella (upper right); B (3000 ×): Intraepithelial DC with a narrow luminal process directly contacting bacteria; note close membrane adhesion to surrounding epithelial cells, focal interruption of the basal lamina (arrow), and outer membrane protein (OMP) immunoreactivity of cytoplasmic vacuoles (b1, 32 000 ×) and a multivesicular late endosome/lysosome body (b2, 22 000 ×); C (8000 ×): DC with clear cytoplasm, close adherence to surrounding epithelial cells, numerous mitochondria, sparse ribosomes and small rough endoplasmic reticulum (RER) cisternae; cytoplasmic vesicles and membranous remnants of an intracellular bacterium, enlarged in c (42 000 ×), show VacA immunoreactivity; D (2000 ×): Nucleated DC with abundant supranuclear mitochondria, no secretory granules, small tubular RER cisternae (enlarged in d1, 18 000 ×), several cytoplasmic vesicles, scattered vacuoles (v), and close adhesion to epithelial cells. On the left, two DC processes (arrows) are contacting the basal membrane (arrowheads). Several cross or longitudinal sections of partly swollen cell processes are observed in the lamina propria; the largest of which (enlarged in d2, 12 000 ×) shows ultrastructural homology with DC cytoplasm, including scattered, small RER cisternae and vesicles; the precise cells of origin of such lamina propria processes could not be assessed. BV: Blood vessel; fg: Foveolar granules; E (2000 ×): Base of foveolar epithelium showing an immature monocytoid cell (DC precursor?) with kidney-shaped nucleus, scattered ribosomes, a few juxtanuclear lysosomes, and no vesicles or granules; note a caliceal process embracing a mast cell (enlarged in e1, 15 000 ×; scroll bodies, arrow), a clubbed process adhering to H. pylori (enlarged in e2, 55 000 ×), a lymphoid cell (Ly) crossing the epithelium basal membrane and another intercellular bacterium (arrow). Reprinted from Necchi et al[73], with permission from John Wiley.
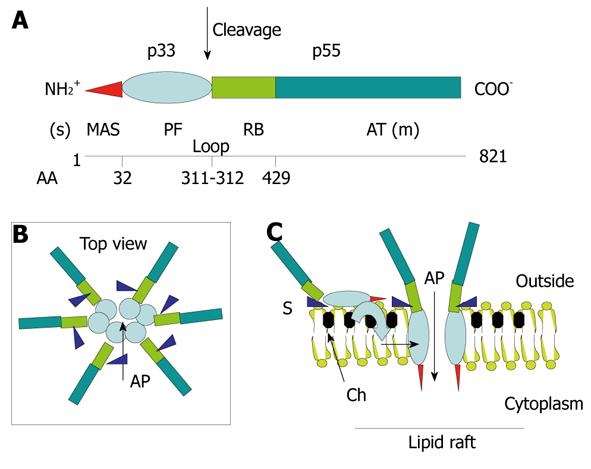
Figure 4 Structure-function relationships in the VacA toxin.
A: VacA is an 88-kDa protein that can be cleaved into two subunits designated p33 (red and light blue) and p55 (light green and dark green). The cleavage between the subunit occurs in a flexible loop between residues 311 and 312. The two subunits are probably attached by non-covalent bonds between the p33 carboxy terminus and the N-p55 terminus. The amino-terminal part of p33 consists of 32 hydrophobic amino-acid stretch that is involved in the recognition of mitochondria (red) (MAS) (where the “s” toxin subtype site is located) followed by the p33 “core” subunit that forms, upon entry into the lipid membrane and oligomerization, an anionic channel (PF) (light blue). The toxin nicking site is located in the flexible loop domain. The amino-terminal part of p55 contains the cell receptor domain (about 110 amino acids) (RB; light green) followed by the type Va autotransporter domain (AT; dark green) that is involved in the secretion of the toxin by the bacterium (where the “m” toxin subtype site is located); B and C: VacA binds as a monomer to its cell surface receptor sphingomyelin (S) with low affinity, then the p33 core progressively is embedded (light blue curve arrow) in the lipid membrane bilayers, at the level of lipid rafts [containing saturated lipid such as sphingomyelin and cholesterol (Ch)] and forms an anionic channel (AP) by oligomerization of p33. This multiplies by 6 the number of toxin cell receptors associated with the oligomerized VacA, thus increasing greatly the toxin affinity for the target cells.
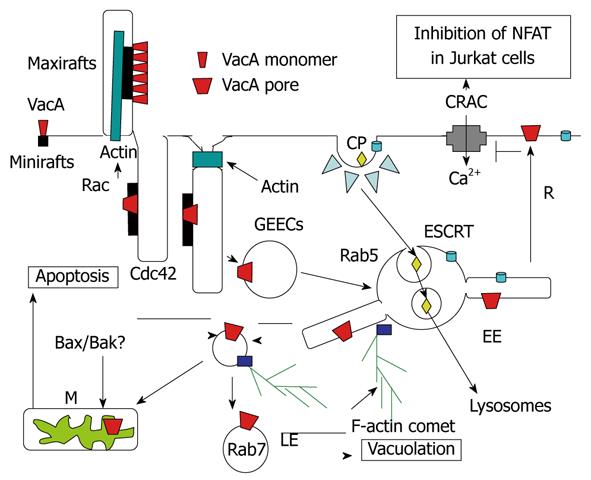
Figure 5 Endocytosis and intracellular trafficking of VacA.
Monomeric VacA may bind sphingomyelin on small rafts. Then, by formation of membrane extensions by actin polymerization via Rac activation, VacA is clustered in macrorafts where the p33 subunit oligomerizes and forms a channel that enters the membrane lipid bilayers. By a Cdc42-dependent process, VacA bound to sphingomelin associated to lipid rafts is transferred into cell membrane invaginations (tubules?), which are then pinched out from the membrane, with the help of F-actin filament, which leading to the formation of the glycosylphosphatidylinositol-anchored protein-enriched early endosomal compartment (GEEC) compartment that contains VacA. The toxin is then transferred to Rab5-positive early endosomes (EEs). In EEs, VacA is selectively addressed to EE tubular extensions that are formed by an F-actin process. These tubular extensions are pinched out from the EEs and form highly motile vacuoles that are propelled by F-actin comets. These motile vesicles then fuse with mitochondria (M) or late endosomes (LEs), where VacA induces apoptosis or vacuolation. The pro-apoptotic channels Bax and Bak may be brought to mitochondria by binding on VacA-containing motile vacuoles. Some VacA molecules may be recycled (R) back to the plasma membrane where the channel activity of the toxin, by altering the electric transmembrane potential, inhibits the voltage-dependent Ca2+ release-activated Ca2+ (CRAC) channel. This blocks entry of calcium that activates the calcineurin protease, which is required for processing of the nuclear factor (NFAT), and therefore inhibits the transcription of the interleukin 2 (IL-2) gene in Jurkat T-lymphocytes. Ligands entering the coated-pit pathway (CP) are directed, by the endosomal sorting complex required for transport (ESCRT) complex, towards internal vesicles of EEs and form the multivesicular body multivesicular body (MVB). MVBs are directed to lysosomes, by being propelled along microtubules, where the contents of EE internal vesicles are transferred and degraded.
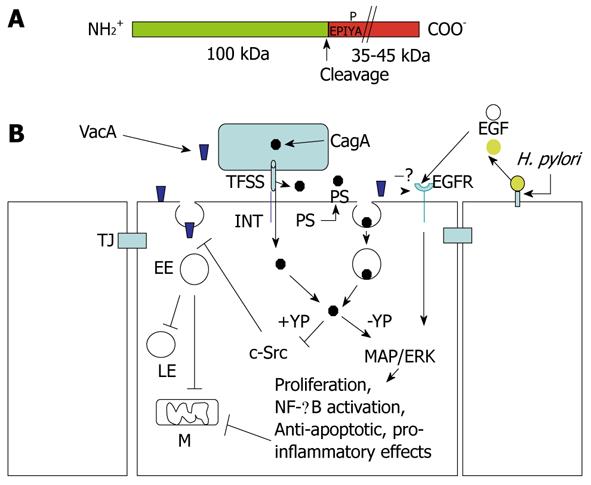
Figure 6 Structure of CagA and signaling cross-talk between VacA and CagA.
A: CagA encompasses two fragments: the N-terminal 100-kDa fragment may contain the cell binding domain [to phosphatidylserine (PS)?]. Cleavage of CagA may take place just at the beginning of the first EPIYA motif, which can be tyrosine-phosphorylated by the c-Src tyrosine kinase. The C-terminal portion of CagA, which contains all the signaling activity of the molecule, may have a different molecular mass (up to 45 kDa) due to the repetition of the EPIYA-containing domain; B: CagA produced within the bacterium is transferred in the external medium by the type IV secretion system (TFSS) machinery. Two possibilities for CagA transfer into the target epithelial cell: (a) by binding to an integrin (INT), the TFSS punches the cell membrane and injects CagA; or (b) the TFSS induces the flipping of PS on the outer cell surface. By its 100-kDa N-terminal fragment, CagA binds PS and, upon endocytosis, which is transferred into the gastric cells. In the cytosol, the CagA signaling domain can be tyrosine-phosphorylated (+YP) and inhibits the c-Src kinase activity that is required to allow the transfer of VacA from glycosylphosphatidylinositol-anchored protein-enriched early endosomal compartments (GEECs) to early endosomes (EEs). This blocks vacuolation in late endosomes (LEs) and mitochondria (M)-dependent apoptosis induced by VacA. In an unphosphorylated (-YP) state, CagA activates mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) and nuclear factor (NF)-κB anti-apototic and pro-inflammatory pathways, which also counteract VacA-induced apoptosis. VacA interferes with epidermal growth factor (EGF) receptor (EGFR) activation and endocytosis, thus impairing the signaling pathway that is triggered by this receptor. Free EGFR ligands (EGF) are liberated from the cell-surface-bound molecules via cleavage triggered by Helicobacter pylori (H. pylori). TJ: Tight junction.
- Citation: Ricci V, Romano M, Boquet P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol 2011; 17(11): 1383-1399
- URL: https://www.wjgnet.com/1007-9327/full/v17/i11/1383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i11.1383