Copyright
©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 7, 2010; 16(45): 5752-5758
Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5752
Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5752
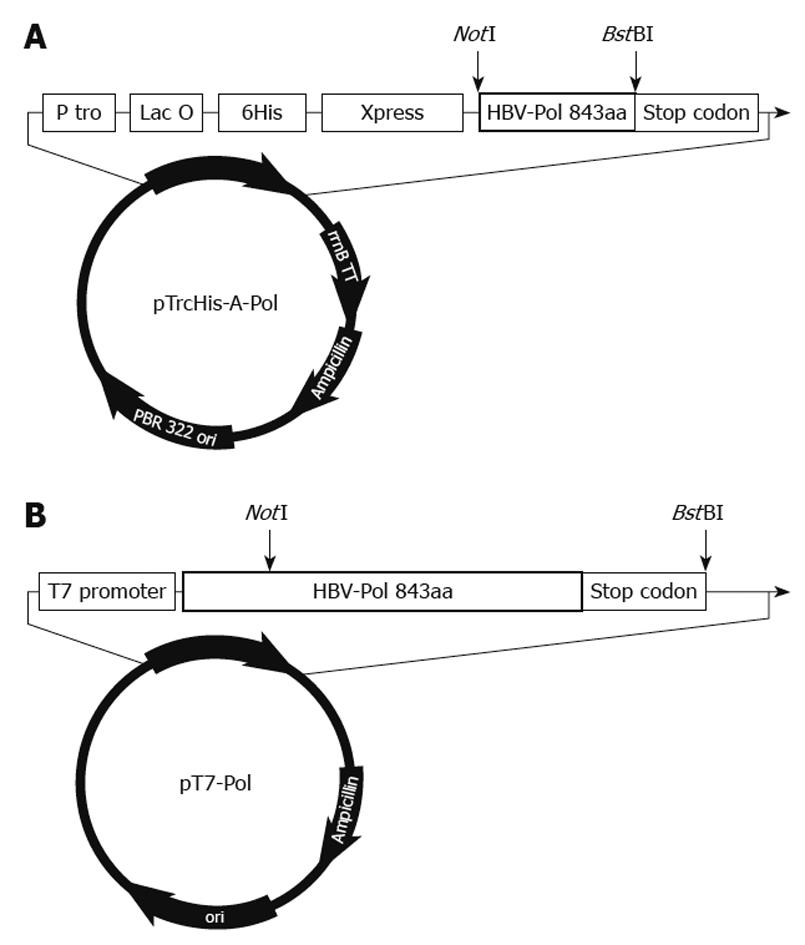
Figure 1 Organization of pTrcHis-A-Pol and pT7-Pol recombinant plasmids.
A: Structural arrangement of pTrcHis-A-Pol. A full-length hepatitis B virus polymerase (HBV-Pol) sequence was fused into pTrcHis-A between NotI and BstBI sites under the control of Trc promoter and Lac operator. This polyhistidine tag plays a role in rapid purification using a nickel-based resin. To determine the expression level under different conditions, an Xpress antigen was fused to the 5’-end of the HBV-Pol sequence; B: Structure of pT7-Pol. A full-length HBV-Pol sequence was fused to P-T7 between NotI and BstBI sites. Full-length HBV-Pol sequence was under the control of the T7 promoter for expression in the TNT T7 transcription–translation-coupled rabbit reticulocyte lysate expression system.
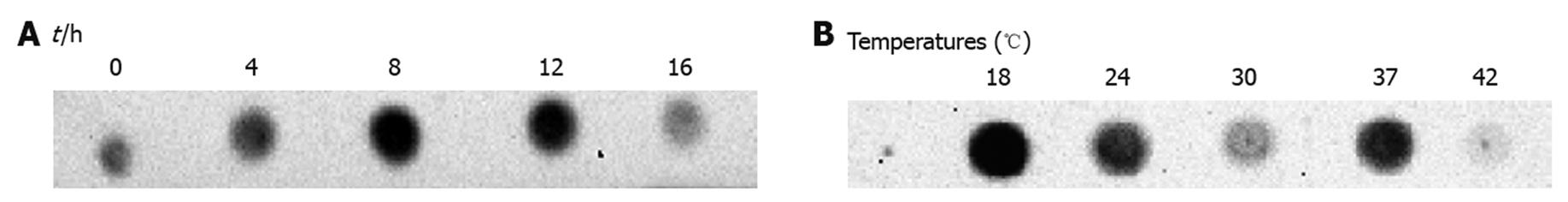
Figure 2 Induction optimization of hepatitis B virus polymerase expressed in Escherichia coli.
Total lysate samples of Escherichia coli transformants after different induction times were blotted on to nitrocellulose membranes. Hepatitis B virus polymerase (HBV-Pol) expression level was determined by anti-Xpress antibody. A: 8-h induction sample showed the strongest signal; B: HBV-Pol expression level at different temperatures was determined by anti-Xpress. Sample from 18°C showed the highest expression level. Preliminary expression data showed that no obvious signal was detected in samples induced at temperatures < 18°C, therefore, previous values for duration and temperature for induction were selected for this study.
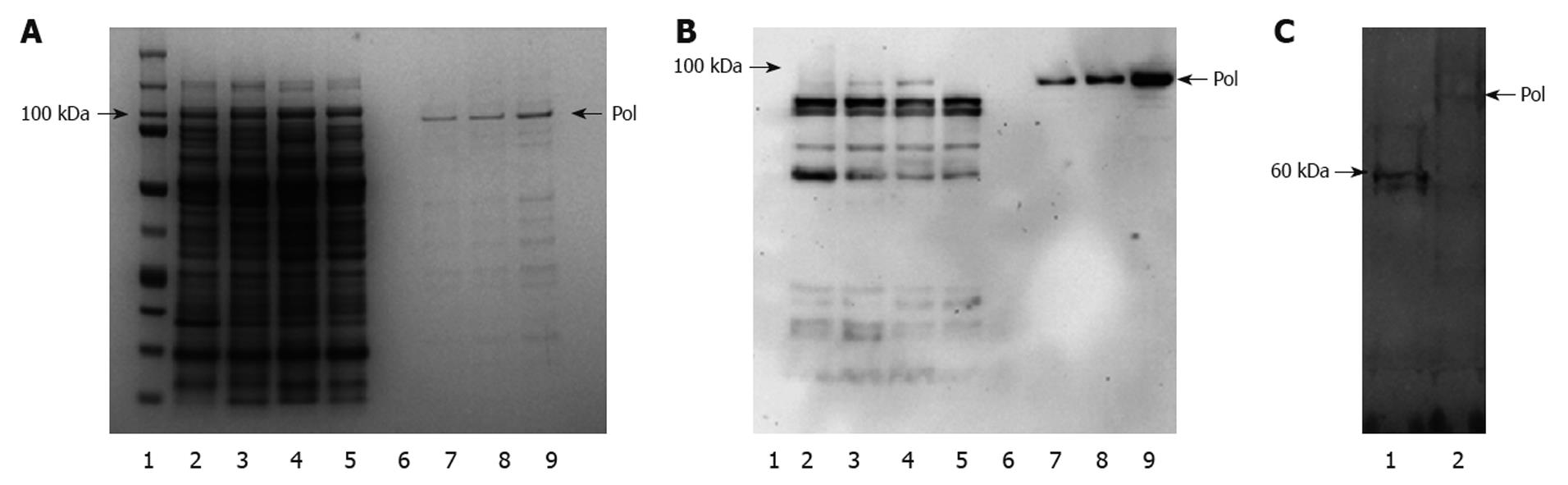
Figure 3 Expression and purification of hepatitis B virus polymerase.
Recombinant human hepatitis B virus polymerase (HBV-Pol) was produced in Escherichia coli (E. coli) cells transformed by pTrcHis-A-Pol and purified with nickel-based resin. Protein samples were analyzed by 4%-12% sodium dodecyl sulfate (SDS)-PAGE, and gels were stained with Coomassie Blue (A). HBV-Pol bands were located at the expected molecular mass, approximate 98 kDa (lane 1). Although HBV-Pol bands were also detected in 50 mmol/L (lane 7) and 75 mmol/L (lane 8) imidazole washing fractions, most HBV-Pol was eluted by 100 mmol/L imidazole (lane 9). Purified recombinant human HBV-Pol was also analyzed by immunoblotting with an anti-reverse transcriptase (RT) antibody (B). HBV-Pol bands were detected in total lysate of uninduced transformant (lane 3) and total lysate of induced transformant (lane 4), but not in untransformed E. coli (lane 2), unbound fraction (lane 5) and fifth washing fraction with 10 mmol/L imidazole (lane 6). Some positive small bands were also detected in lanes 2-5, which was mainly because some components from total lysate of E. coli were recognized by anti-RT polyclonal antibody. Expression of human HBV-Pol in TNT T7 transcription-translation-coupled rabbit reticulocyte lysate expression system is shown in C. PT7-pol and pT7-luciferase (1650 bp, ORF size approximate 61 kDa) were added to in vitro transcription-translation reactions for 75 min at 30°C in the presence of [35S]-methionine. Five microliters of each sample was incubated in SDS sample buffer at 95°C for 3 min, and electrophoresed through 4%-12% SDS-PAGE. A band of pT7-luciferase (lane 1) and pT7-pol (lane 2) were detected by autoradiography.
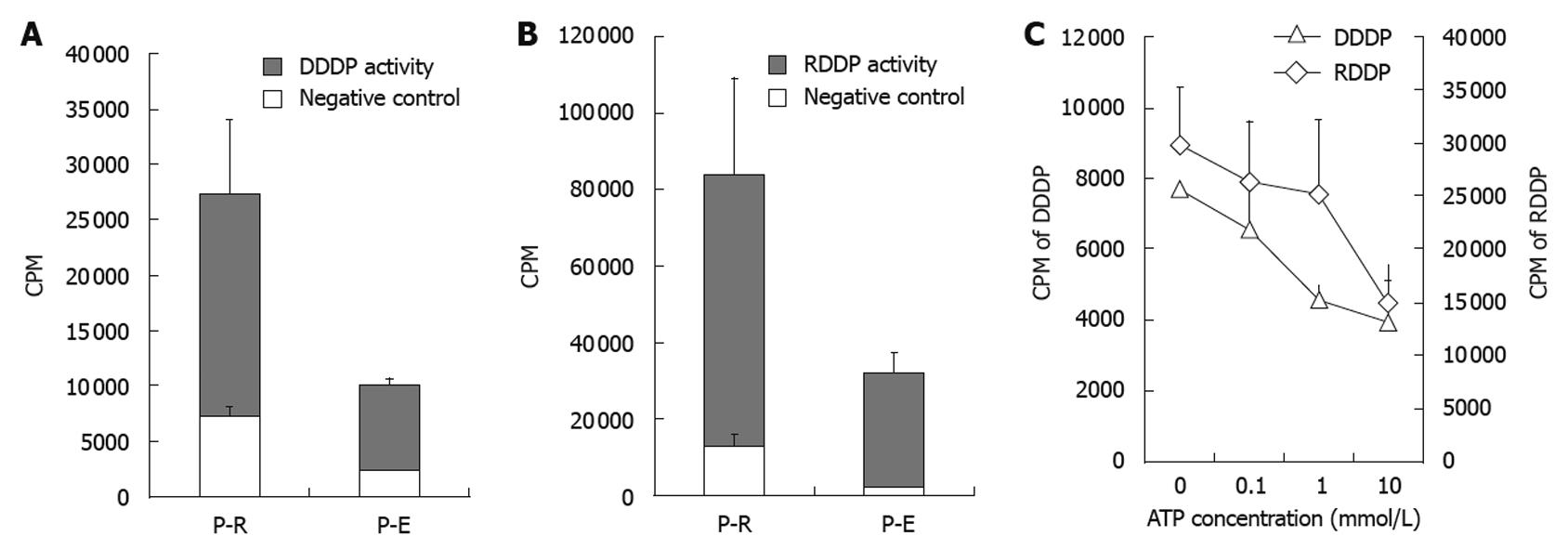
Figure 4 Enzyme activity assay of recombinant hepatitis B virus polymerase.
Comparison of enzyme activities between hepatitis B virus polymerase (HBV-Pol) from Escherichia coli expression system (P-E) and in vitro expression system (P-R). DNA-dependent DNA polymerase (DDDP) activity and RNA-dependent DNA polymerase (RDDP) activity of the P-E and P-R were monitored under standard conditions. All endogenous DDDP activities were suppressed by 60 mmol/L aphidicolin and 1 mmol/L NEM (A and B). The effects of increasing concentrations of adenosine triphosphate (ATP) are shown in C. DDDP activity using poly (dA) • oligo (dT)12-18 and RDDP activity using poly (rA) • oligo (dT)12-18 were used as the substrate for DDDP activity and RDDP activity assays, respectively. CPM: Counts per minute.
- Citation: Yu Y, Pandeya DR, Liu ML, Liu MJ, Hong ST. Expression and purification of a functional human hepatitis B virus polymerase. World J Gastroenterol 2010; 16(45): 5752-5758
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5752