Copyright
©2010 Baishideng.
World J Gastroenterol. Aug 7, 2010; 16(29): 3664-3673
Published online Aug 7, 2010. doi: 10.3748/wjg.v16.i29.3664
Published online Aug 7, 2010. doi: 10.3748/wjg.v16.i29.3664
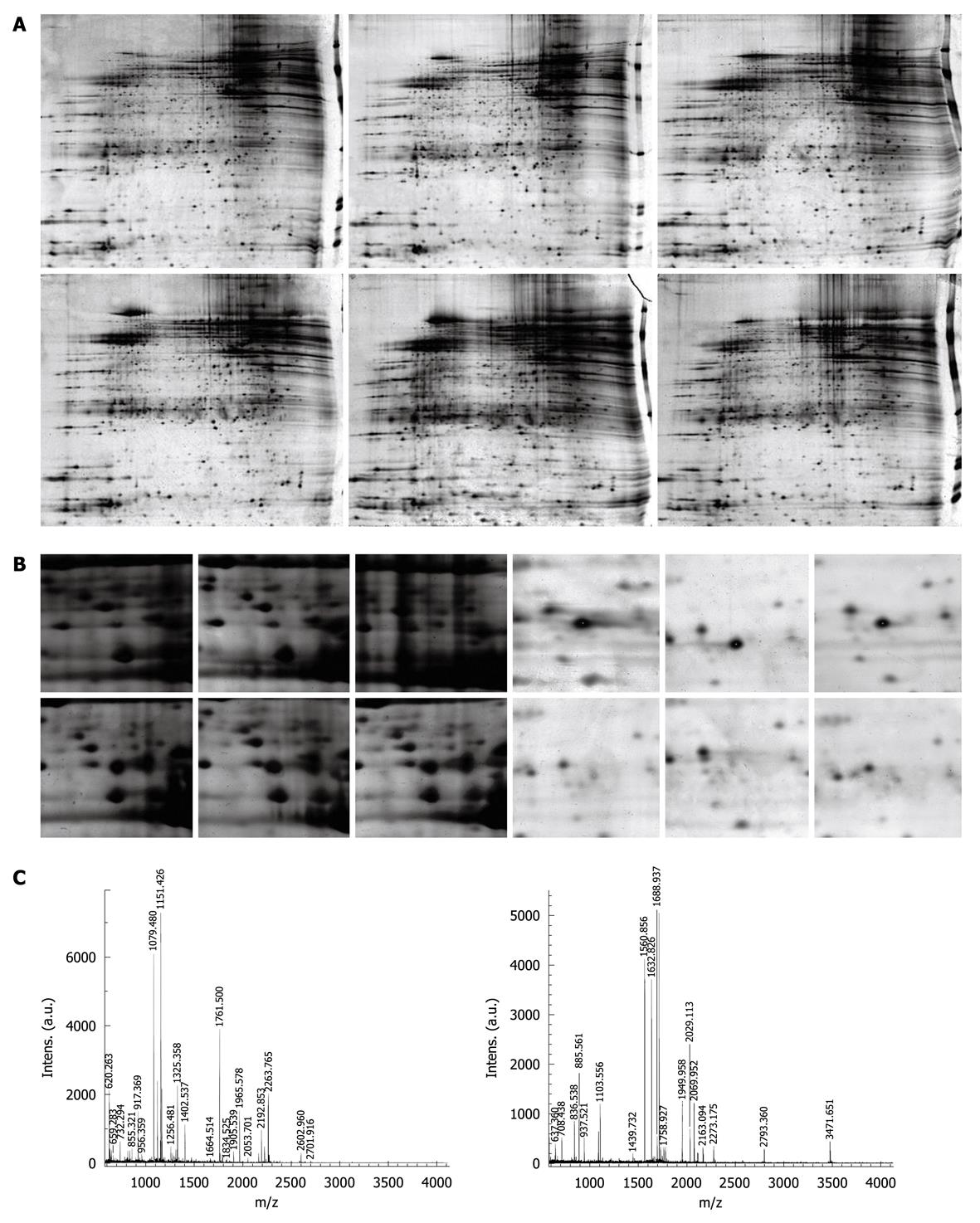
Figure 1 Detection and analysis of differentially expressed proteins in varioliform gastritis.
A: Representative 2-DE images of matched varioliform gastritis and normal gastric mucosa tissue. The proteins expressed in varioliform gastritis were compared with those expressed in matched normal tissue. The protein spots that showed more than 5-fold differential expression in at least nine cases were taken as differentially expressed candidates. Of 21 differentially expressed protein spots, 18 were identified by mass spectrometry (MS) (protein nomenclature can be seen in Table 2); B: The magnified regions of the 2-DE gel of upregulated thioredoxin domain-containing protein 5 (TXNDC5) (left) and downregulated phosphatidylethanolamine-binding protein 1 (PEBP1) (right) in varioliform gastritis, compared with normal tissue; C: MS of in-gel trypsin digests of these proteins and analysis of the depicted peptide spectrum resulted in the identification of TXNDC5 (left) and PEBP1 (right).
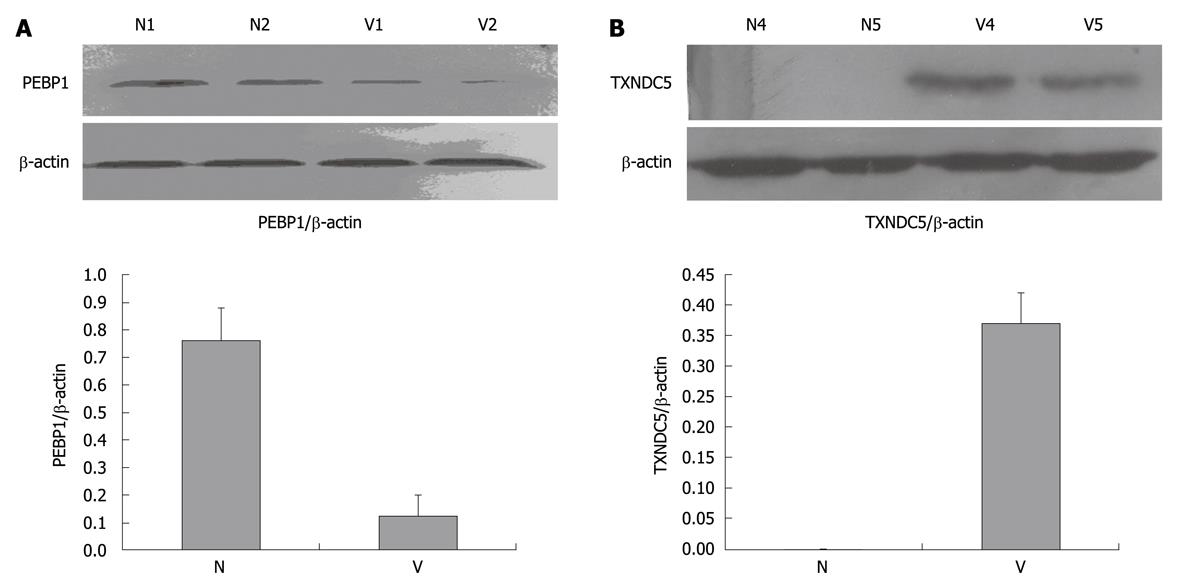
Figure 2 Western blotting analysis of phosphatidylethanolamine-binding protein 1 and thioredoxin domain-containing protein 5.
A: Marked downregulation of phosphatidylethanolamine-binding protein 1 (PEBP1) in varioliform gastritis (V) tissue. Protein extracts (50 μg) were separated on a 12% sodium dodecyl sulfate-polyacrylamide gel. Proteins were transferred to a poly-vinylidene difluoride membrane. After blocking, the membranes were incubated with rabbit monoclonal antibody of PEBP1 (dilution of 1:2000) and subsequently incubated with HRP-anti-rabbit IgG. The specific proteins were visualized with chemiluminescent reagent. As a control for equal protein loading, blots were restained with anti-actin antibody. Immunosignals were quantified by densitometry scanning. The relative quantification was calculated as the ratio of PEBP1 expression to actin expression as shown in the followed chart; B: Upregulation of thioredoxin domain-containing protein 5 (TXNDC5) in varioliform gastritis in comparison with that in normal (N) mucosa. The same experimental process was performed, except that the membranes were incubated with polyclonal goat anti-TXNDC5 antibody (dilution of 1:1000).
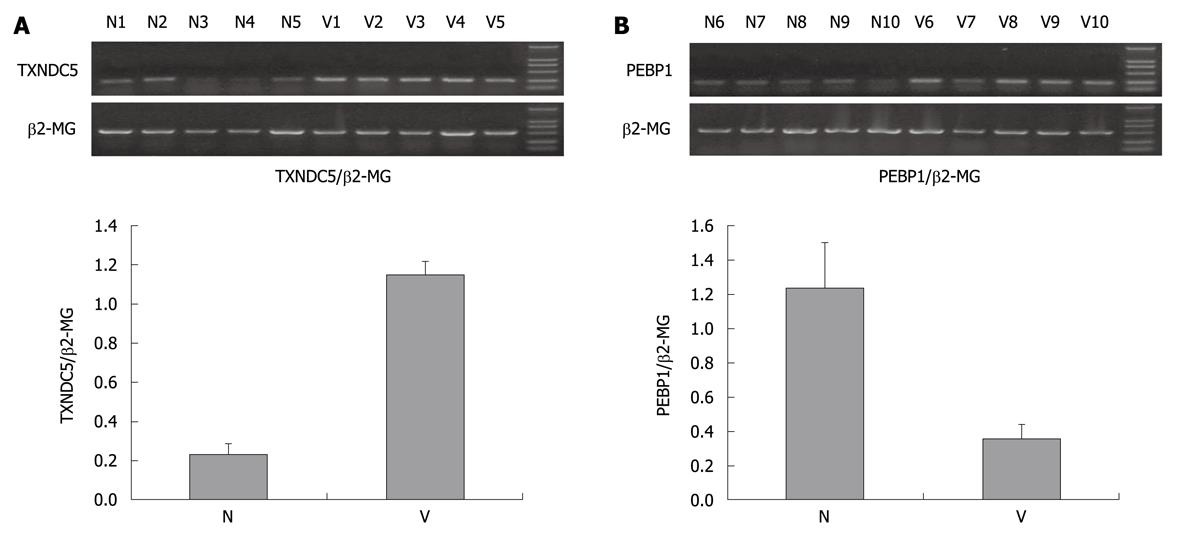
Figure 3 Reverse transcription polymerase chain reaction analysis of thioredoxin domain-containing protein 5 and phosphatidylethanolamine-binding protein 1.
A: Marked upregulation of thioredoxin domain-containing protein 5 (TXNDC5) in varioliform gastritis (V). The total RNAs of additional samples tissues were extracted by homogenization in Trizol (Invitrogen), cDNA synthesis was performed in 20 μL reaction system of reverse transcription including 5 μg RNA. Amplification of TXNDC5, with β2-MG acting as internal control, was carried out in DNA thermal cycler. PCR products were separated by 1.5% agarose gel electrophoresis. The bands were quantified by densitometry scanning. The relative quantification was calculated as the ratio of TXNDC5 expression to β2-MG expression as shown in the followed chart; B: Downregulation of phosphatidylethanolamine-binding protein 1 (PEBP1) in varioliform gastritis in comparison with that in normal (N) mucosa. The same experimental process was performed. The relative quantification was calculated as the ratio of PEBP1 expression to β2-MG expression as shown in the followed chart.
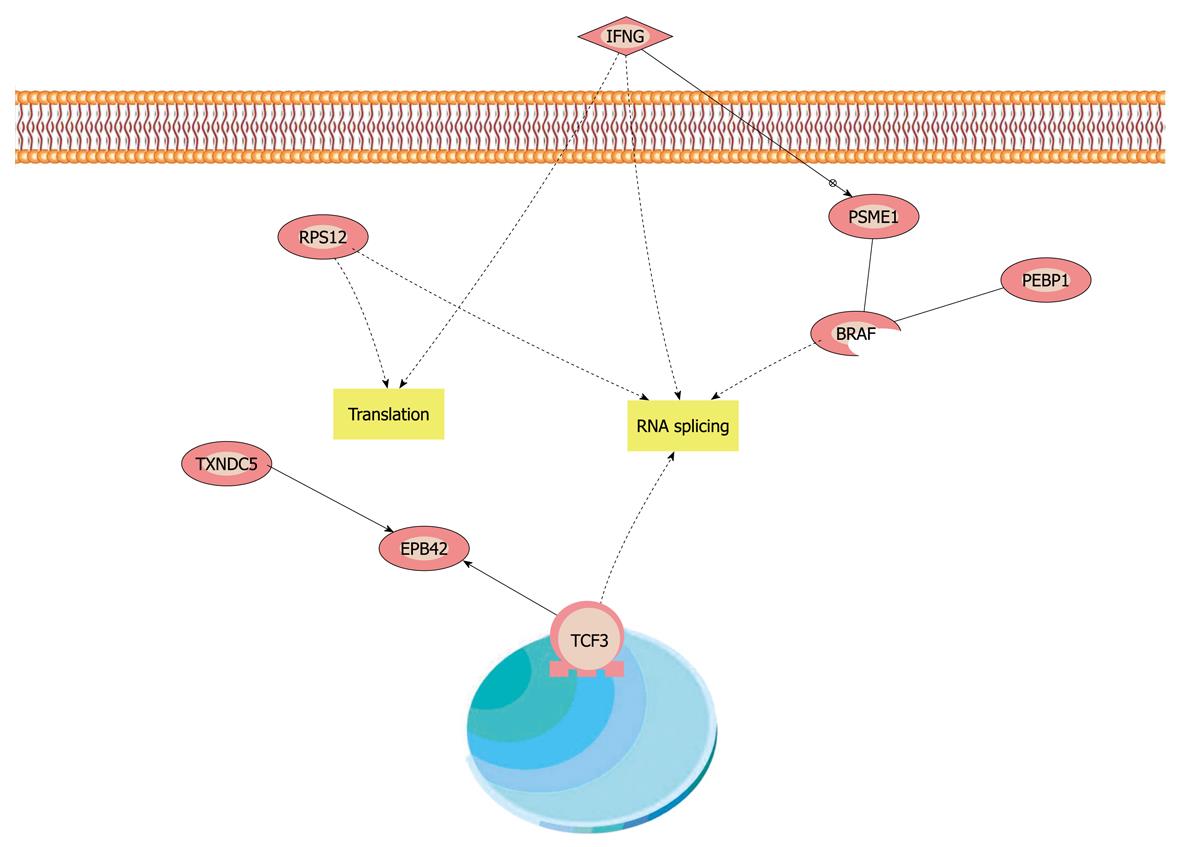
Figure 4 Compendious biological interaction networking of phosphatidylethanolamine-binding protein 1 and thioredoxin domain-containing protein 5 in varioliform gastritis.
Phosphatidylethanolamine-binding protein 1 (PEBP1) and thioredoxin domain-containing protein 5 (TXNDC5) were imported into Pathway Studio (demo), and an interaction map was generated. Compendious molecular interaction pathway which linked PEBP1, TXNDC5 and interaction pathways and interferon γ (INFG). INFG could induce some complex molecular interaction in cells and impact on cell proliferation and apoptosis, and then could promote the formation of varioliform lesion.
-
Citation: Zhang L, Hou YH, Wu K, Zhai JS, Lin N. Proteomic analysis reveals molecular biological details in varioliform gastritis without
Helicobacter pylori infection. World J Gastroenterol 2010; 16(29): 3664-3673 - URL: https://www.wjgnet.com/1007-9327/full/v16/i29/3664.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i29.3664