Copyright
©2010 Baishideng.
World J Gastroenterol. Jul 7, 2010; 16(25): 3120-3132
Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3120
Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3120
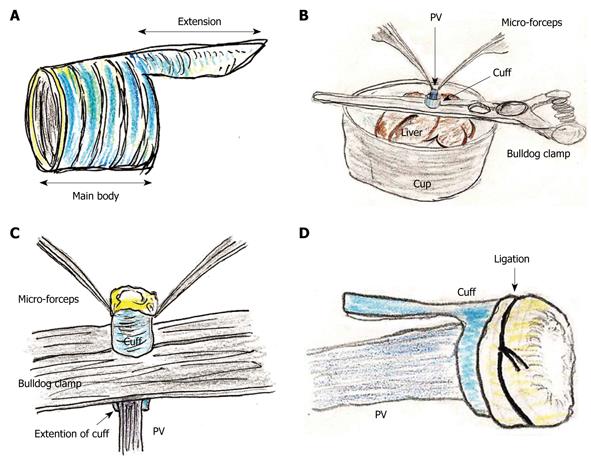
Figure 1 Portal vein (PV) cuff attachment.
A: Cuff body with encircled chases and extension are made; B: PV trunk is induced through PV cuff. Cuff extension and PV trunk are grasped with a straight large-sized bulldog clamp. Cuff is set on the cup; C: Wall of PV trunk completely reversed using micro-forceps; D: Reversed PV wall is fixed to chase on cuff by ligation of silk thread.
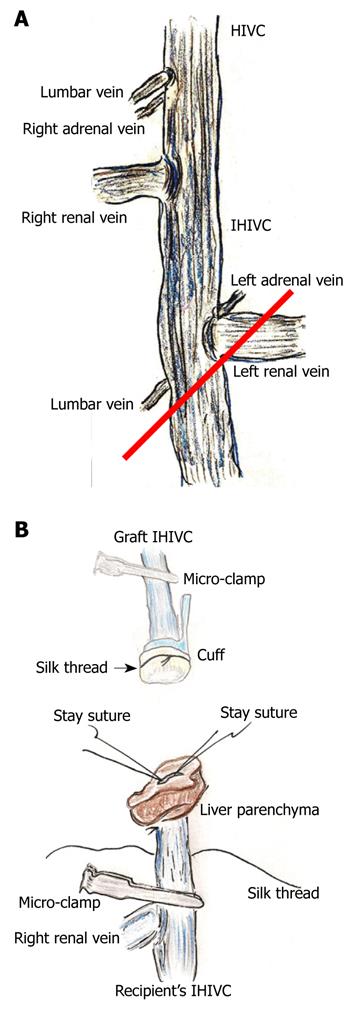
Figure 2 Infra-hepatic inferior vena cava (IHIVC) branches and reconstruction.
A: Right adrenal and lumbar veins flow directly into IHIVC at point of lowest edge of right inferior segment. Left adrenal and the lower lumbar veins flow into the IHIVC at the junction of the left renal vein and the IHIVC. In donor operation for IHIVC reconstruction using the cuff method, IVIHC is cut in a branch patch-fashion (red line); B: Inner side of recipient’s IHIVC is easily detected by adherent liver parenchyma. Stay sutures are made bilaterally on posterior wall, and anterior wall has some allowance for cuff insertion. Stay sutures are held by a bulldog clamp and are pulled to the cranial side. Silk thread is set behind the recipient’s IHIVC beforehand. Confirmation of quality of graft IHIVC is confirmed using saline flush. Cuff is led towards the recipient’s IHIVC, and cuff is inserted into IHIVC.
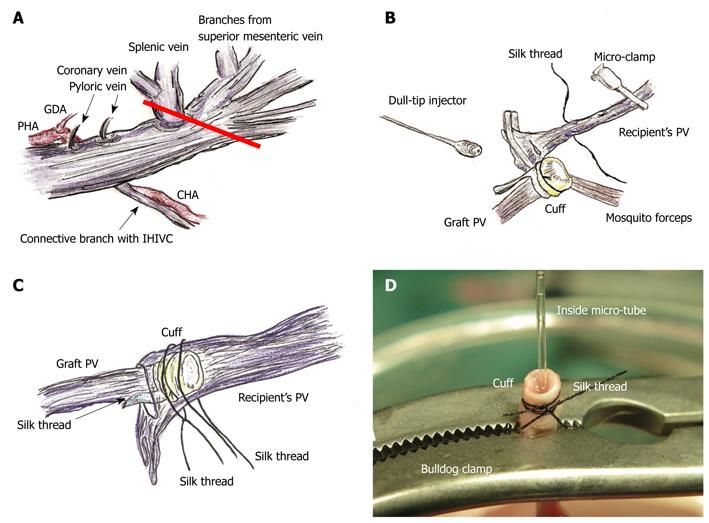
Figure 3 PV preparation and reconstruction.
A: The splenic vein and coronary vein flow into PV trunk at the left or posterior sides of the PV. The pyloric vein flows into PV trunk at the left side of PV. The posterior side of PV trunk has a branch that connects with the IHIVC. The common hepatic artery (CHA) is located at the back of PV trunk, and the proper hepatic artery (PHA) and gastroduodenal artery branches are located at the left side of PV trunk. The PV trunk and these arteries are encased together in a thin sheath. The PV trunk is cut in a branch patch-fashion using PV trunk and splenic vein in the donor operation (red line); B: Retention of recipient PV is performed using mosquito forceps. Silk thread is set behind the recipient’s PV trunk beforehand. The natural form of the PV is confirmed using saline flush. The cuff is led onto recipient’s PV. The PV is opened using the cut-down method at the point nearest the hepatic hilus, and patency of the inner side is confirmed by saline flush; C: Cuff is inserted into recipient’s PV avoiding any torsion; D: At the back table a bulldog clamp holds PV trunk, micro-tube, and cuff extension. Because of the micro-tube inside, detection of the inner side is simple. GDA: Gastroduodenal artery.
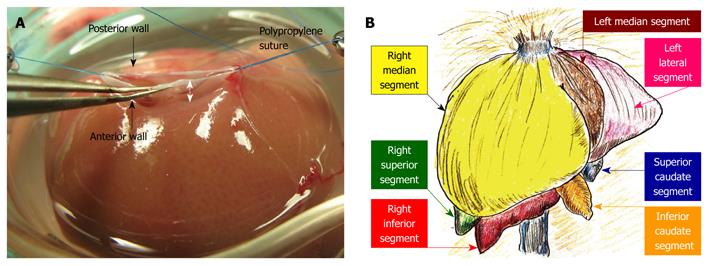
Figure 4 Hepatic segments and hepatic vein flows.
A: The hepatic vein itself has no extra-hepatic margins for suture. The most important techniques for SHIVC plasty are (1) ensuring enough margin of the wall, and (2) retention using stay sutures. In particular, sufficient margin of the SHIVC wall (white arrow) is indispensable for confirmation of optimal out-flow; B: The liver comprises 3 lobes, which are subdivided into 7 segments. In basic anatomy, the left median segment is joined with the right median segment, and an incomplete lobulation is often detected in those segments.
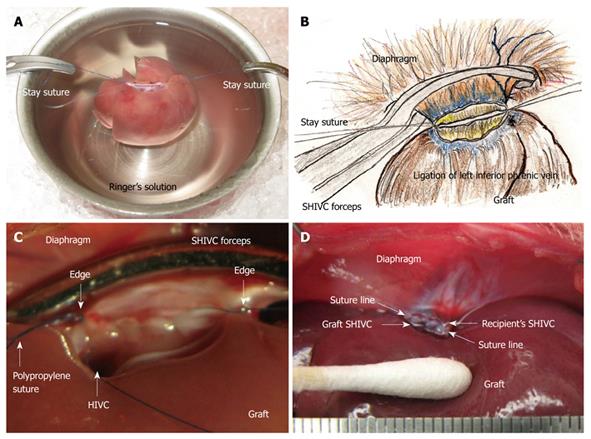
Figure 5 Supra-hepatic inferior vena cava (SHIVC) reconstruction.
A: Note that an out-flow block can make the model unusable, and points of stay sutures without the axis torsion should be checked again before allograft implantation; B: The stay suture is placed bilaterally after careful consideration of the setup. The posterior wall straightens and the anterior wall is set as an arch; C: Finding after suture of the posterior wall is shown. HIVC is confirmed to enter into the right lobe. Too tight a ligation causes stenosis of the HIVC and disturbance of the flow. Ligations with stay sutures on both sides should be completed, although not too tightly; D: Completed SHIVC reconstruction is shown.
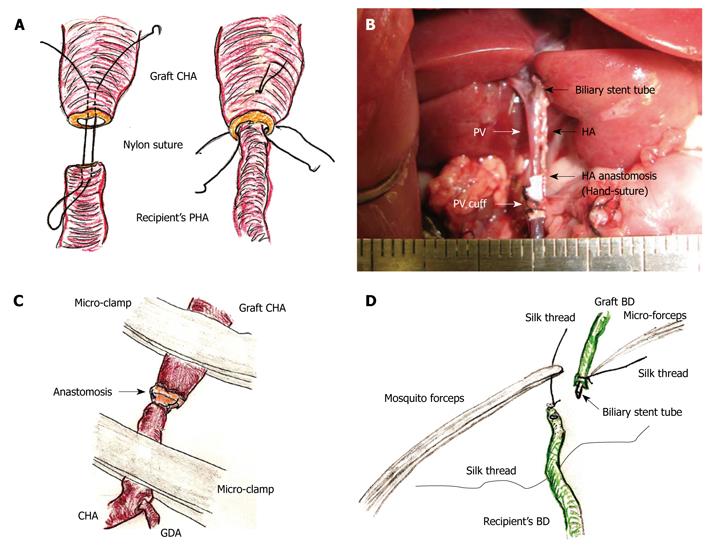
Figure 6 Hepatic artery (HA) and biliary duct (BD) reconstruction.
A: An initial suture is made through the whole layer of the CHA from the outside to the inside, and a thrusting is then performed through the whole layer of the PHA from the inside to the outside. Subsequently, reverse thrusting from the PHA to the CHA is performed with the same thread. The recipient’s PHA is then led into the graft CHA. One or two superficial stitches can be added if bleeding occurs; B: The diameter of the recipient’s PHA is approximately 0.2 mm. Complete ultra-microsurgery allows the use of end-to-end anastomosis in HA reconstruction; C: Intermittent and alternate clamping of the HA also achieves hemostasis; D: The previously ligated silk thread of the recipient’s BD is held using mosquito forceps. The recipient’s BD is encircled beforehand with silk thread. The biliary stent tube is led into the recipient’s BD. The recipient’s BD is open using the cut-down method, and the stent tube is inserted.
Figure 7 Learning curve in a surgeon.
The initial 50 trial surgeries by one surgeon are shown. Learning curves of each surgeon showed similar patterns. Whole liver grafts were used in all cases. Preservation solution and cold ischemic time are unified as Ringer’s solution for 2 h.
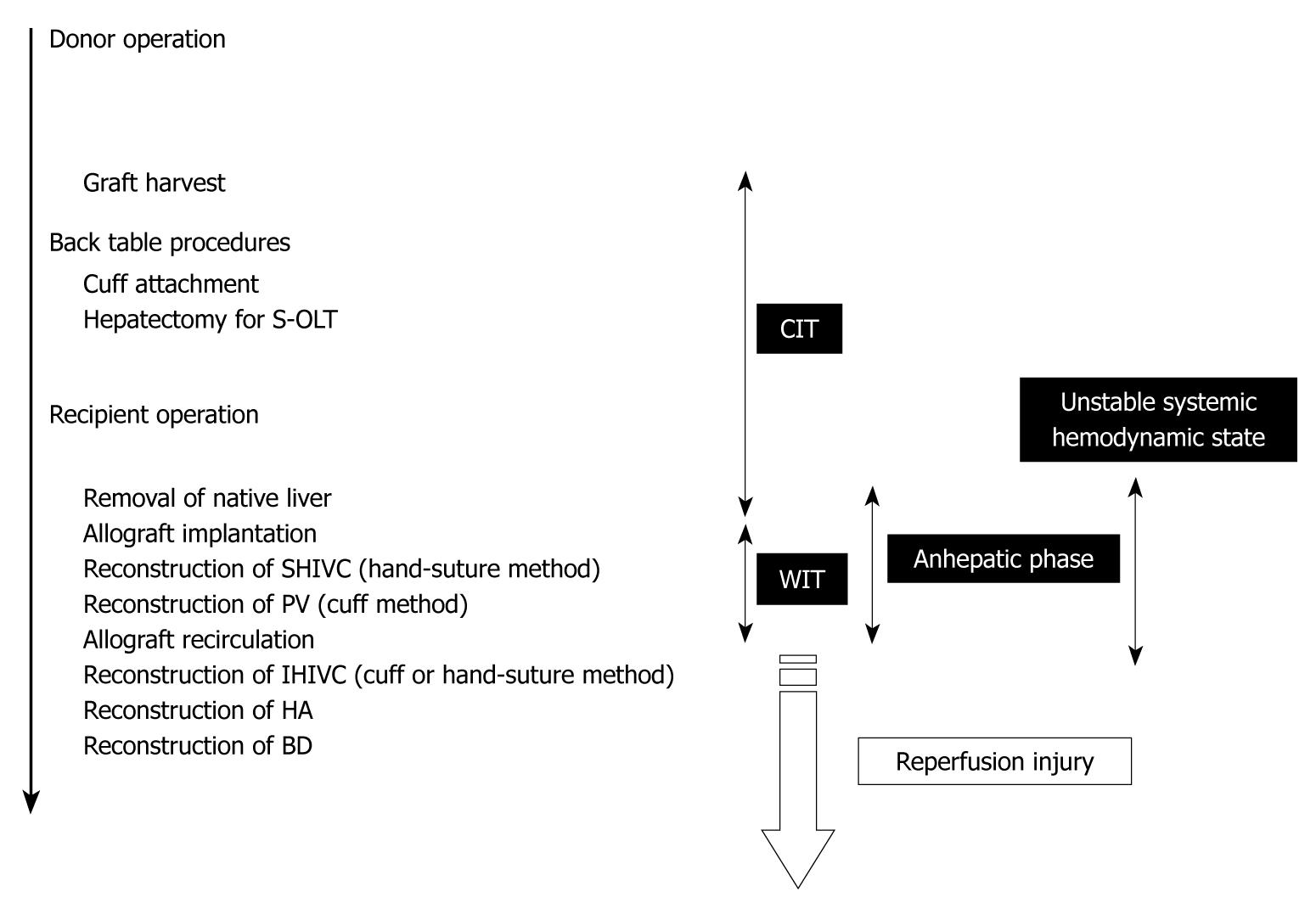
Figure 8 Time table of OLT in rat.
OLT: Orthotopic liver transplantation; CIT: Cold ischemic time; WIT: Warm ischemic time.
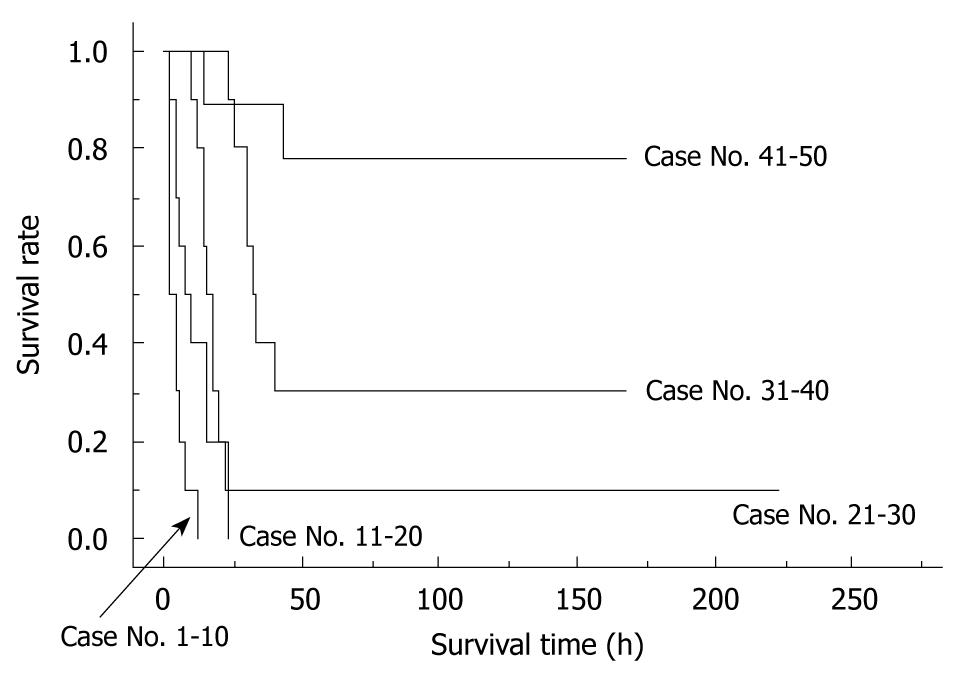
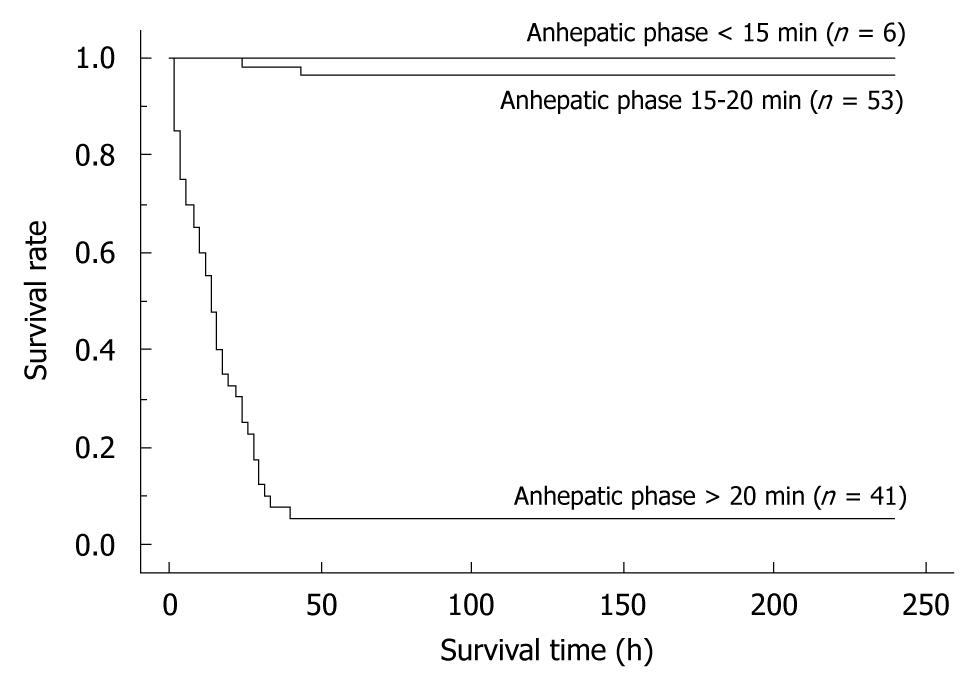
Figure 9 The differences in survival rates based on the anhepatic phase length.
Recipients with a long anhepatic phase had poor survival rates, while recipients with an anhepatic phase of 15-20 min often died due to surgical issues. In contrast, recipients with an anhepatic phase < 15 min all survived. Whole liver grafts were used in all cases. All cases were accompanied by HA reconstruction. Preservation solution and cold ischemic time are unified as the Ringer’s solution for 2 h. Anhepatic phase < 15 min vs anhepatic phase 15-20 min: P = 0.0830; Anhepatic phase < 15 min vs anhepatic phase > 20 min: P < 0.0001.
- Citation: Hori T, Nguyen JH, Zhao X, Ogura Y, Hata T, Yagi S, Chen F, Baine AMT, Ohashi N, Eckman CB, Herdt AR, Egawa H, Takada Y, Oike F, Sakamoto S, Kasahara M, Ogawa K, Hata K, Iida T, Yonekawa Y, Sibulesky L, Kuribayashi K, Kato T, Saito K, Wang L, Torii M, Sahara N, Kamo N, Sahara T, Yasutomi M, Uemoto S. Comprehensive and innovative techniques for liver transplantation in rats: A surgical guide. World J Gastroenterol 2010; 16(25): 3120-3132
- URL: https://www.wjgnet.com/1007-9327/full/v16/i25/3120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i25.3120