Copyright
©2010 Baishideng.
World J Gastroenterol. Jun 14, 2010; 16(22): 2743-2753
Published online Jun 14, 2010. doi: 10.3748/wjg.v16.i22.2743
Published online Jun 14, 2010. doi: 10.3748/wjg.v16.i22.2743
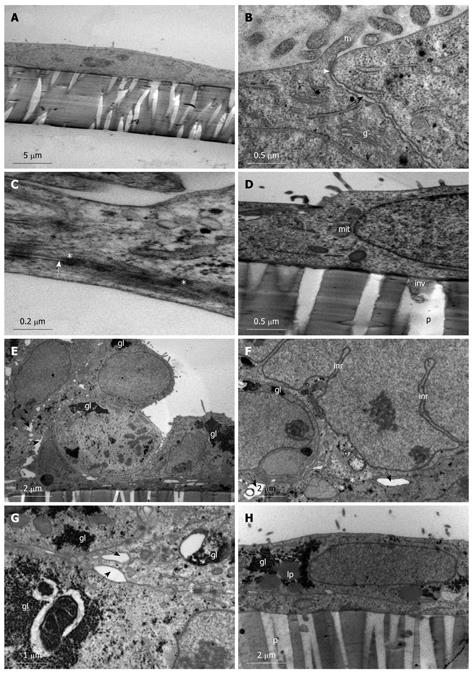
Figure 1 Spontaneous differentiation of Caco-2 into sub-population 1 of stratified cuboidal cells.
A-D: Caco-2 cells grown on membrane filters examined on day 1 one post-confluency. A: Simple squamous cells with irregular sparse microvilli; B: Junction between two cells (black arrow) and establishment of a tight junction (white arrow) and the presence of microvilli (m) and golgi bodies (g); C: Desmosome (*) formation along the lateral plasma membrane between two cells (white arrow); D: Basolateral plasma membrane depicting attachment to membrane filter with evidence of small cell protrusions (inv) into the membrane pore (p). Mitochondria (mit); E-H: Caco-2 cells grown on membrane filters examined 12 d post-confluency. E: Cells appear stratified and cuboidal with large intercellular spaces (black arrows); large glycogen stores are evident (gl); F: Intercellular spaces (black arrows) and desmosomes (*) are evident between cells with glycogen deposits (gl) and indentations of the nucleus form large intranuclear rods (inr); G: High power image of intercellular spaces (black arrows) and desmosomes (*) present along the lateral plasma membrane with large glycogen deposits (gl) found in the cytoplasm; H: Basolateral plasma membrane showing attachment of the cell to the membrane filter. No invasive processes are evident projecting into the filter pores (p). Glycogen (gl) and some lipid (lp) droplets are present in the cell cytoplasm.
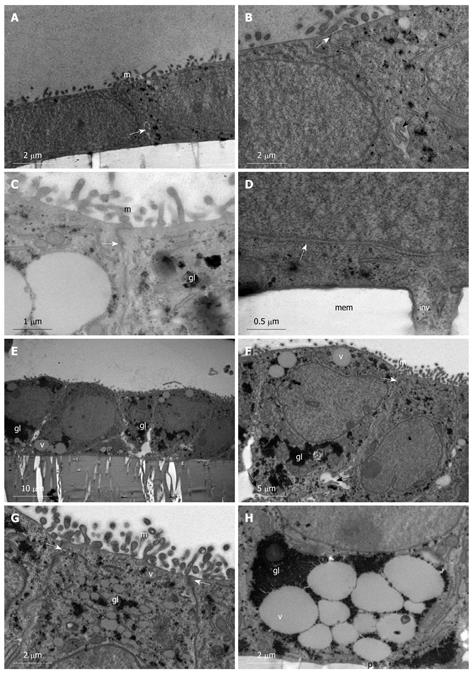
Figure 2 Spontaneous differentiation of Caco-2 into sub-population 2 of simple columnar monolayer of polarized epithelial cells.
A-D: Caco-2 cells grown on membrane filters examined on day 1 post-confluency. A: Simple cuboidal epithelium with regular microvilli (m) and intercellular junctions (white arrow); B: Intercellular junction with a tight junction (white arrow) evident along the apical part of the lateral plasma membrane. A small intercellular space (black arrow) is evident near the basolateral region of the plasma membrane; C: High power image of a tight junction (white arrow) between cells showing glycogen particles (gl) and microvilli (m); D: Basolateral plasma membrane showing intercellular junction (white arrow) and small invasive projection (inv) into the filter pore. Membrane (mem); E-H: Caco-2 cells grown on membrane filters examined on day 12 post-confluency. E: Simple columnar polarized epithelium is present with large glycogen stores (gl), mucin filled vacuoles (v), and intercellular spaces along the basal part of the lateral plasma membrane; F: Junction between two columnar cells (white arrow); regular microvilli (m) are present as well as large glycogen stores (gl) and mucin filled vacuoles (v). Intercellular space (black arrow); G: High power micrograph showing the apical domain of cells with regular microvilli (m), glycogen stores (gl), mucin vacuoles (v), and tight junction formation (white arrows); H: High power image of the basal domain of cells with large mucin filled vacuoles (v) enclosed by large glycogen stores (gl). No invasive processes are evident projecting into filter pores (p).
Figure 3 Undifferentiated heterogenous population of HT-29 cells.
A-D: HT-29 cells grown on membrane filters (mem) and examined day 1 post-confluency. A: Stratified cuboidal cells arranged in clumps. Large intercellular spaces (black arrows) are evident with microvilli projecting into them; B: High power micrograph showing large intercellular spaces (approximate 2 μm) (white arrows) with microvillar projections and formation of small vacuoles (v); C: Microvillous (m) apical plasma membrane is present and desmosomes (*) are evident along contact sites of the lateral plasma membrane of adjoining cells and presence of intercellular spaces (white arrow); D: No invasive projections are evident along the basolateral plasma membrane. Desmosomes (*) are present at contact sites of the plasma membrane, which otherwise shows intercellular spaces (white arrow); E-H: HT-29 cells grown on membrane filters (mem) and examined day 12 post-confluency. E: Stratified cuboidal cells with microvillar projections entering the large intercellular space (black arrow). Intranuclear rods are evident (inr); F: High power micrograph of junction between two cells. Golgi bodies (g) as well as mucin filled vacuoles (v) are distributed throughout the cell cytoplasm and intercellular spaces are evident with microvilli projecting into their lumen; G: High power micrograph of a contact site between two opposing plasma membranes showing a desmosome (*). Large intercellular spaces (black arrows) are evident above and below the desmosomes contact site. Mitochondria (mit) and mucin filled vacuoles (v) are also evident; H: Basolateral attachment site showing minor invasive projections (inv) into the filter pore. Intercellular spaces (black arrow) and vacuoles (v) are evident.
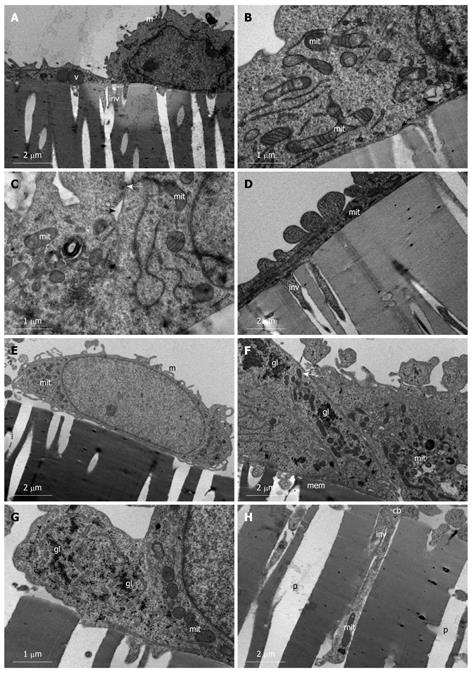
Figure 4 Undifferentiated heterogenous population of SW480 cells.
A-D: SW480 cells grown on membrane filters and examined day 1 post-confluency. A: Simple squamous (left) and simple cuboidal (right) distinctions are already evident in cells on day 1 post-confluency. Cells exhibit vacuoles (v), microvilli (m), and invasive processes (inv) are evident projecting into filter pores; B: Large population of mitochondria (mit) are evident which exhibit branching characteristics; C: Intercellular junctions (black arrow) exhibit early formation of the tight junction (white arrow). Mitochondria (mit) with numerous cristae are present; D: Simple squamous cells exhibiting long invasive processes (inv) into the filter pores. Mitochondria (mit); E-H: SW480 cells grown on membrane filters and examined day 12 post-confluency. E: Cells remain a monolayer with microvillar (m) projections and abundant mitochondria (mit); F: Junction between two adjoining cells growing on membrane (mem) showing no intercellular spaces, tight junction formation (white arrow), abundant mitochondria (mit), and glycogen (gl) stores; G: High power micrograph depicting the glycogen (gl) granules in the cytoplasm in amongst mitochondria (mit); H: Basolateral region of cell body (cb) showing deep invasive processes (inv) projecting into filter pores (p). These processes extend to depths of approximately 10 μm and cellular organelles such as mitochondria (mit) are present in these processes, showing deep anchorage of cell.
-
Citation: Biazik JM, Jahn KA, Su Y, Wu YN, Braet F. Unlocking the ultrastructure of colorectal cancer cells
in vitro using selective staining. World J Gastroenterol 2010; 16(22): 2743-2753 - URL: https://www.wjgnet.com/1007-9327/full/v16/i22/2743.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i22.2743