Copyright
©2009 The WJG Press and Baishideng.
World J Gastroenterol. Feb 14, 2009; 15(6): 648-674
Published online Feb 14, 2009. doi: 10.3748/wjg.15.648
Published online Feb 14, 2009. doi: 10.3748/wjg.15.648
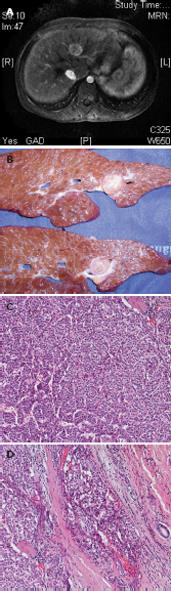
Figure 1 Adolescent affected by tyrosinemia who developed hepatocellular carcinoma, despite 2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione therapy.
A: Magnetic resonance imaging displays a 26-mm lesion. B: After liver transplantation, the resected liver showed multiple nodules in the left lobe. C: Histological sections from the nodule revealed hepatocellular carcinoma. D: Microvascular invasion.
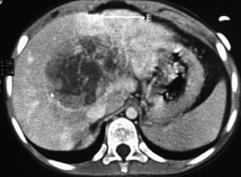
Figure 2 Non-resectable hepatoblastoma.
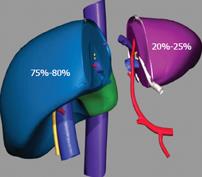
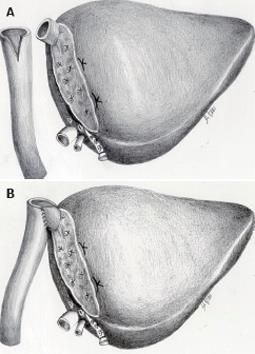
Figure 3 Spit liver allows for the procurement of two separate grafts of different size.
A section of the liver is made along the falciform ligament and divides the left lateral segment from the extended right liver. The left graft, composed of segments 2 and 3, and representing 20%-25% of the total liver volume, includes the left hepatic vein, the left branch of the portal vein, and the left branch of the hepatic artery, along with the common hepatic artery and the celiac tripod. The right graft composed of segments 1 and 4-8, and representing 75%-80% of the total liver volume, includes the vena cava, the right branch of the hepatic artery, and the portal vein.
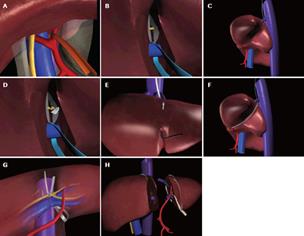
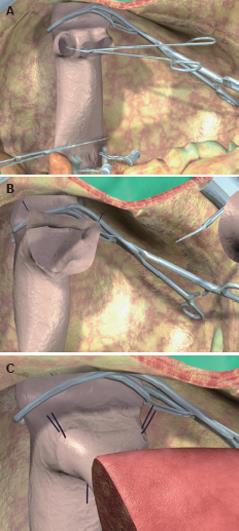
Figure 4 Main phases of split liver procurement.
A: Dissection of the hepatogastric ligament and encircling of the left hepatic artery; B: Identification and encircling of the extrahepatic portion of the left branch of the portal vein; C: Isolation and encircling of the left hepatic vein; D: Division with a scalpel of the porta hepatis containing the bile duct(s) of the left lateral segment; E: Section of the parenchyma started along the falciform ligament; F: Identification of the plane of parenchymal dissection by passing the cotton tape, which encircled the left hepatic vein, on the posterior surface of the liver in the fossa of the ductus venosus; G: Laterally to the left branch of the hepatic artery and of the portal vein; H: The two partial grafts at the end of the procedure.
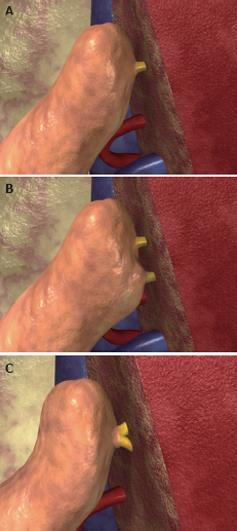
Figure 5 Anastomosis between the left hepatic vein of the graft and the inferior vena cava of the recipient, performed with the triangulation technique.
A: The bridge between the ostia of the right, middle, and left hepatic veins is cut to obtain a single opening; B: The opening is further enlarged by cutting the anterior face of the vena cava to obtain a wide triangular orifice; C: Three 5/0 vascular monofilament sutures are placed, taking the three corners of the graft and recipient orifices.
Figure 6 Biliary reconstruction performed by means of hepatico-jejunostomy.
The bile duct of the graft can be single or double, although in the latter case, two different anastomoses are performed (B) or, if the two ducts are closed sufficiently, a common orifice can be created and anastomosed to the bowel loop (C).
Figure 7 Use of left lateral segment grafts to transplant children affected by hepatic malignancies, with replacement of the recipient’s inferior vena cava using an iliac vein graft from the donor.
On the back table a wide V-shaped opening on the wall of the common iliac vein graft from the donor is made (A), and the left hepatic vein of the left lateral segment graft is anastomosed end-to-side to the V-shaped opening on the common iliac vein (B).
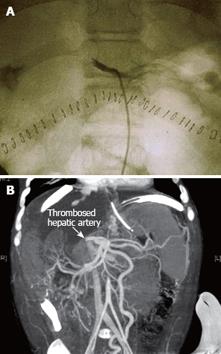
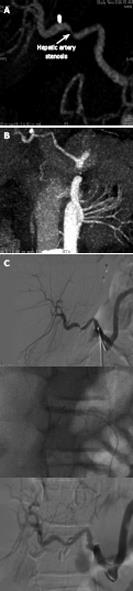
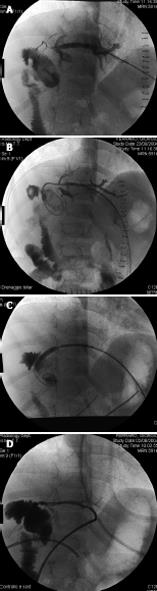
Figure 8 Selective celiac angiography showing early hepatic artery thrombosis after left lateral segment transplantation.
A: Conventional angiography is the gold standard for radiographic diagnosis of hepatic artery thrombosis. B: Nowadays, the sensitivity of multiphase, multislice computed tomographic angiography with multidetector reconstruction approaches that of conventional angiography.
Figure 9 A case of hepatic artery stenosis.
Reconstructed computed tomographic angiography demonstrating severe hepatic artery stenosis in an extended right graft recipient (A), and complete resolution of the stenosis 6 mo later (B), after stenosis treatment by early interventionally guided balloon angioplasty (C).
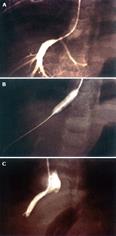
Figure 10 Venogram of hepatic venous outflow obstruction after left lateral segment split-liver transplantation.
Venogram demonstrates a stenosis at the left hepatic vein anastomosis (A). Balloon angioplasty is performed (B), with resolution of the stenosis (C).
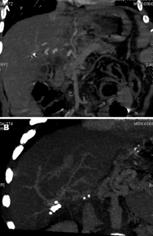
Figure 11 Portal vein thrombosis.
Computed tomographic angiography with evidence of portal vein thrombosis and cavernomatous degeneration with collateral drainage through the left gastric vein (A), and evidence of intrahepatic portal flux restoration (B).
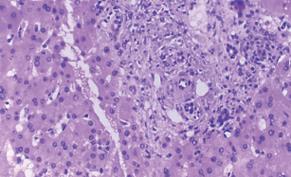
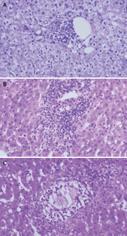
Figure 12 Liver biopsy performed in a left lateral segment recipient because of non-specifically elevated liver function tests.
Histology shows biliary duct proliferation and portal tract enlargement suggestive of mechanic cholestasis.
Figure 13 A case of biliary stenosis.
Percutaneous transhepatic cholangiography performed in a left lateral segment recipient demonstrating intrahepatic biliary tree dilatation with stenosis of the hepaticojejunostomy (A), balloon bilioplasty (B), and transanastomotic percutaneous transhepatic biliary drainage positioning (C). Resolution of the stenosis after three sessions of bilioplasty (D).
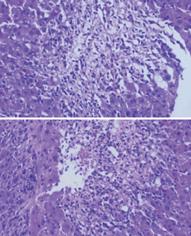
Figure 14 Acute cellular rejection: histopathological findings and grading.
A: Mild acute cellular rejection, portal tracts are mildly expanded because of a predominantly mononuclear, but mixed portal inflammation. Rejection infiltrate is composed of blastic and small lymphocytes, eosinophils, macrophages, and occasional plasma cells. Lymphocytes are also present inside the basement membrane of the small bile ducts and in the subendothelial space of small portal vein branches. B: Moderate acute cellular rejection, all the portal tracts are markedly expanded by a predominantly mononuclear, but mixed inflammation. Centrilobular inflammation and hepatocyte necrosis and dropout are absent. C: evere acute cellular rejection, severe expansion of the portal tracts because of inflammation with focal portal-to-portal bridging; perivenular inflammation with hepatocyte necrosis and dropout; inflammation and damage to small bile ducts.
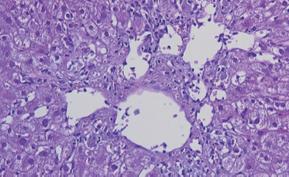
Figure 15 Histological findings in chronic rejection: Little portal inflammation in conjunction with bile duct loss affecting > 50% of the portal tracts and moderate or severe perivenular fibrosis.
Figure 16 Histological appearance of recurrent or new-onset autoimmune hepatitis characterized by moderate portal inflammation, prominent interface activity, relative sparing of the bile ducts, and perivenular accumulation of inflammation.
- Citation: Spada M, Riva S, Maggiore G, Cintorino D, Gridelli B. Pediatric liver transplantation. World J Gastroenterol 2009; 15(6): 648-674
- URL: https://www.wjgnet.com/1007-9327/full/v15/i6/648.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.648