Copyright
©2009 The WJG Press and Baishideng.
World J Gastroenterol. Jan 21, 2009; 15(3): 310-320
Published online Jan 21, 2009. doi: 10.3748/wjg.15.310
Published online Jan 21, 2009. doi: 10.3748/wjg.15.310
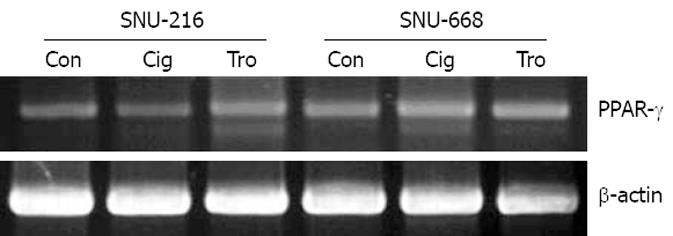
Figure 1 PPAR-γ expression was confirmed by RT-PCR in human gastric cancer cell lines (SNU-216 and SNU-668) treated with troglitazone (Tro) or ciglitazone (Cig) and the β-actin control (Con) is shown in the bottom panel.
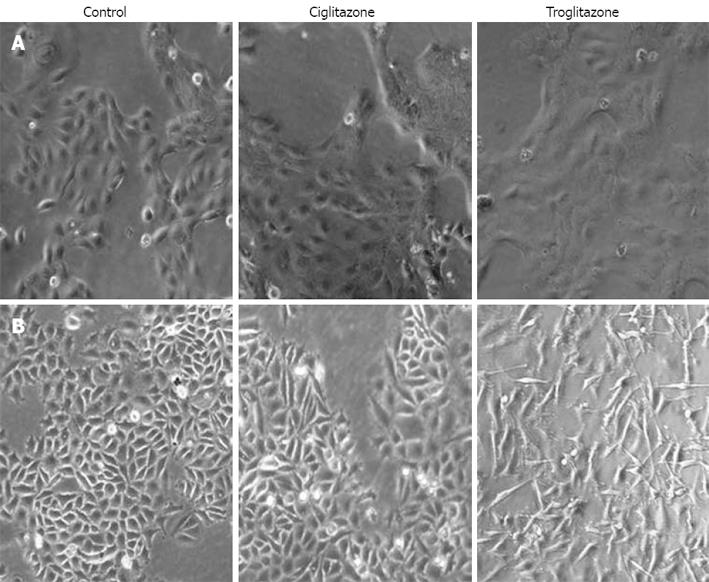
Figure 2 Change in cell morpho-logy after treatment with troglita-zone or ciglitazone.
There were more significant differences in SNU-668 (B) than SNU-216 (A) cells, as shown by inverted microscopy (original magnification, x 100).
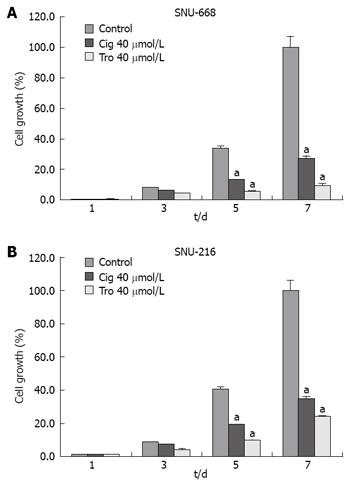
Figure 3 Growth inhibition by troglitazone or ciglitazone in human gastric cancer cell lines.
There was a more significant increase of suppressive effect in SNU-668 (A) than SNU-216 (B) cells compared with the control group. aP < 0.05 vs control group.
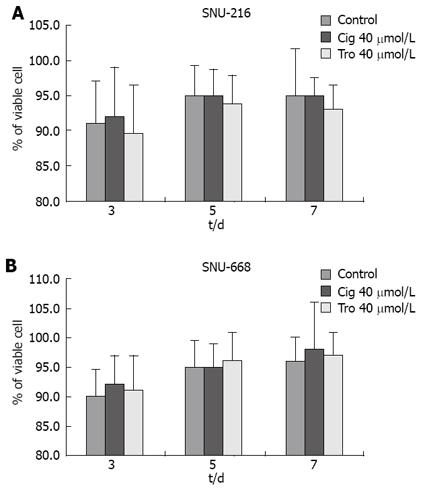
Figure 4 Cell viability measured by hemocytometry.
Viability of the troglitazone- or ciglitazone-treated cells was decreased more than that of the control group, with no significant difference between the two cell lines.
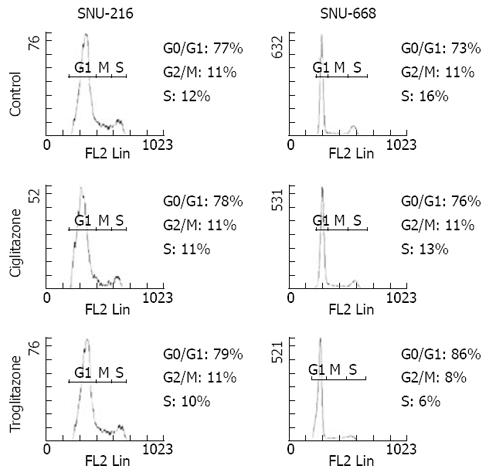
Figure 5 Effects of troglitazone and ciglitazone on cell cycle distribution measured by flow cytometry.
It shows meaningful arrest during G2/M and S phase in SNU-668 treated with triglitazone.
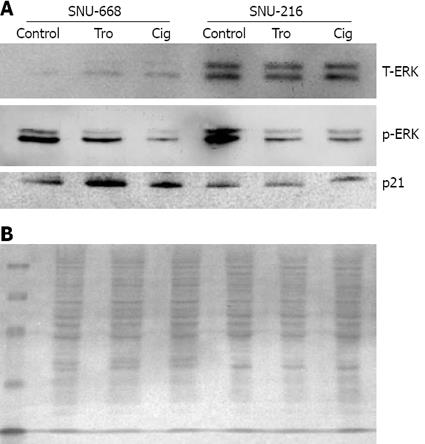
Figure 6 Western blot analysis for the expression of total-ERK, p-ERK and p21.
A: There was a significant decrease in phosphorylation of ERK and increased expression of p21 in SNU-668 cells with ciglitazone or troglitazone treatment. B: Ponceau S protein staining of the membrane of SNU-216 and SNU-668 cells to ensure equal loading of protein in the sample.
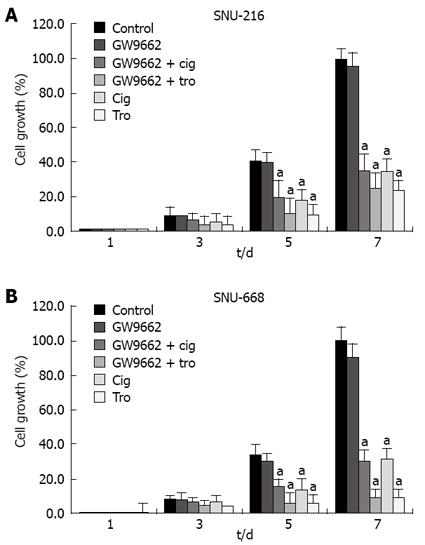
Figure 7 Effect of GW9662 on the inhibition of cell proliferation.
GW9662, a selective PPAR-γ suppressor, had no effect on ciglitazone- or troglitazone-induced inhibition of cell proliferation. aP < 0.05 vs control group.
- Citation: Cheon CW, Kim DH, Kim DH, Cho YH, Kim JH. Effects of ciglitazone and troglitazone on the proliferation of human stomach cancer cells. World J Gastroenterol 2009; 15(3): 310-320
- URL: https://www.wjgnet.com/1007-9327/full/v15/i3/310.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.310