Copyright
©2008 The WJG Press and Baishideng.
World J Gastroenterol. Feb 14, 2008; 14(6): 864-875
Published online Feb 14, 2008. doi: 10.3748/wjg.14.864
Published online Feb 14, 2008. doi: 10.3748/wjg.14.864
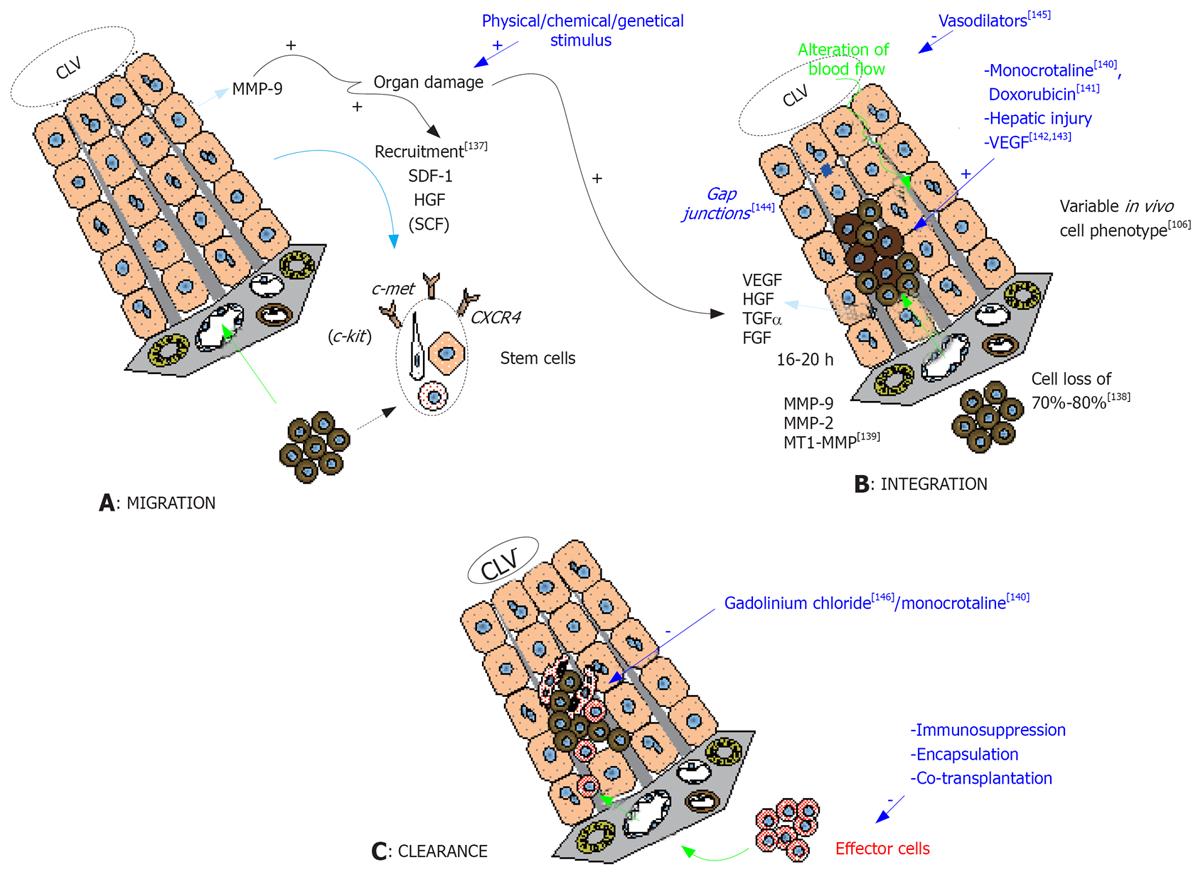
Figure 1 Mechanisms of liver repopulation after stem cell transplantation.
A: Stem cell migration into the liver parenchyma after spleen infusion is guided mainly by the splenic and the portal vein flow. Homing of the cells is tightly regulated by chemoattractive agents (as SDF-1, HGF and SCF) secreted by liver cells under liver damage conditions[136], and acting respectively through the interaction with CXCR4, c-met and c-kit receptors of stem cells. MMP-9, expressed by host hepatocytes after injury, is implicated in the homing process through its action on the extracellular matrix. This process can be triggered by various types of liver injury; B: While reaching the portal vein, 70% to 80% of the infused cells are lost and phagocytosis by macrophages/phagocytes can be observed in the portal areas[137]. After 16-20 h post-infusion, the remaining cells enter the liver sinusoid after having crossed over the endothelial barrier rendered permeable by a local secretion of VEGF[137], and by the combined action of various MMPs[138]. The permeabilization of the endothelium by specific drugs owning toxicity against endothelial cells (as monocrotaline[139] or doxorubicin[140]) or acting on the permeation (VEGF[141142]) was described to enhance the repopulation rate of the infused cells. The penetration of the parenchyma is facilitated by the action of MMPs and the disruption of gap junctions[143]. The cell implantation creates an alteration of venous blood flow which can hamper the progression of the cells inside the parenchyma but can be alleviated by the use of vasodilators[144]. Globally, inducing liver injury facilitates cell integration by remodeling the liver architecture and by creating a local microenvironment favorable for the proliferation of the transplanted cells. This is mediated by the local secretion of cytokines/growth factors (HGF, FGF, TGFα). Cell implantation implicates the reformation of gap junctions that can be observed 3 to 7 d after transplantation. The implanted cells display variable cell phenotype parallel to the level of their hepatocyte differentiation; C: After integration, some cells will be cleared from the hepatic parenchyma. Dead cells lacking implantation stimulus or cell-to-cell interactions will be phagocytes by Kupffer cells. This can be inhibited by the use of gadolinium chloride[145] or by monocrotaline[139] which was demonstrated in animal models to improve the kinetics of cell implantation. Other cells will be rejected by the host immunological system (effector cells) and tools to alleviate this rejection are multiple (immunosuppression protocols, encapsulation of the infused cells, co-transplantation with cells owning immunosuppressive properties). FGF: Fibroblastic growth factor; HGF: Hepatocyte growth factor; MMP: Matrix metalloproteinase; SCF: Stem cell factor; SDF: Stromal derived factor; TGF: Transforming growth factor; VEGF: Vascular endothelial growth factor.
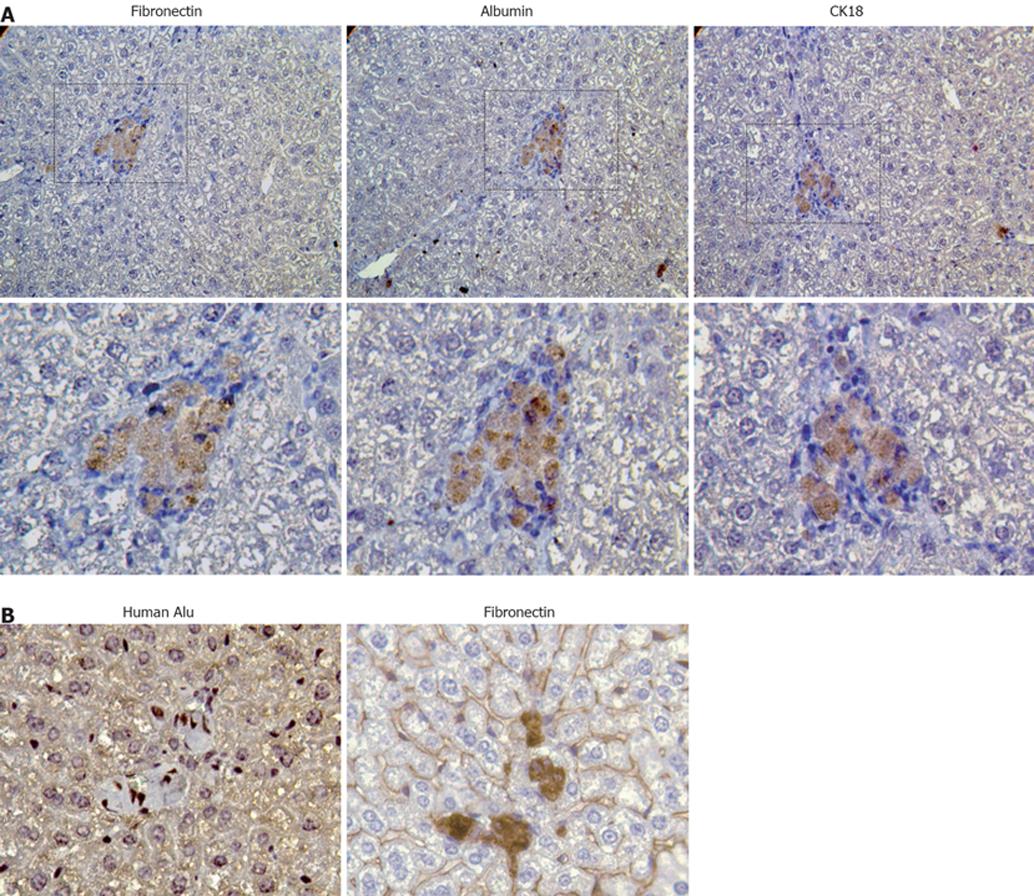
Figure 2 Analysis of liver engraftment of transplanted stem cells.
Human BM-MSCs were transplanted into the liver of partially hepatectomized SCID mice and livers were screened after one month for analysis. A: Staining on serial sections for fibronectin, albumin and CK18 by immunohistochemistry revealed a co-expression of mesodermal and hepatocyte markers and confirmed the chimerical phenotype of hepatocyte differentiating MSCs (400 ×); B: The use of combined techniques for revealing the expression of human alu probe by in situ hybridization and of fibronectin by immunohistochemistry in a cell cluster (800 ×).
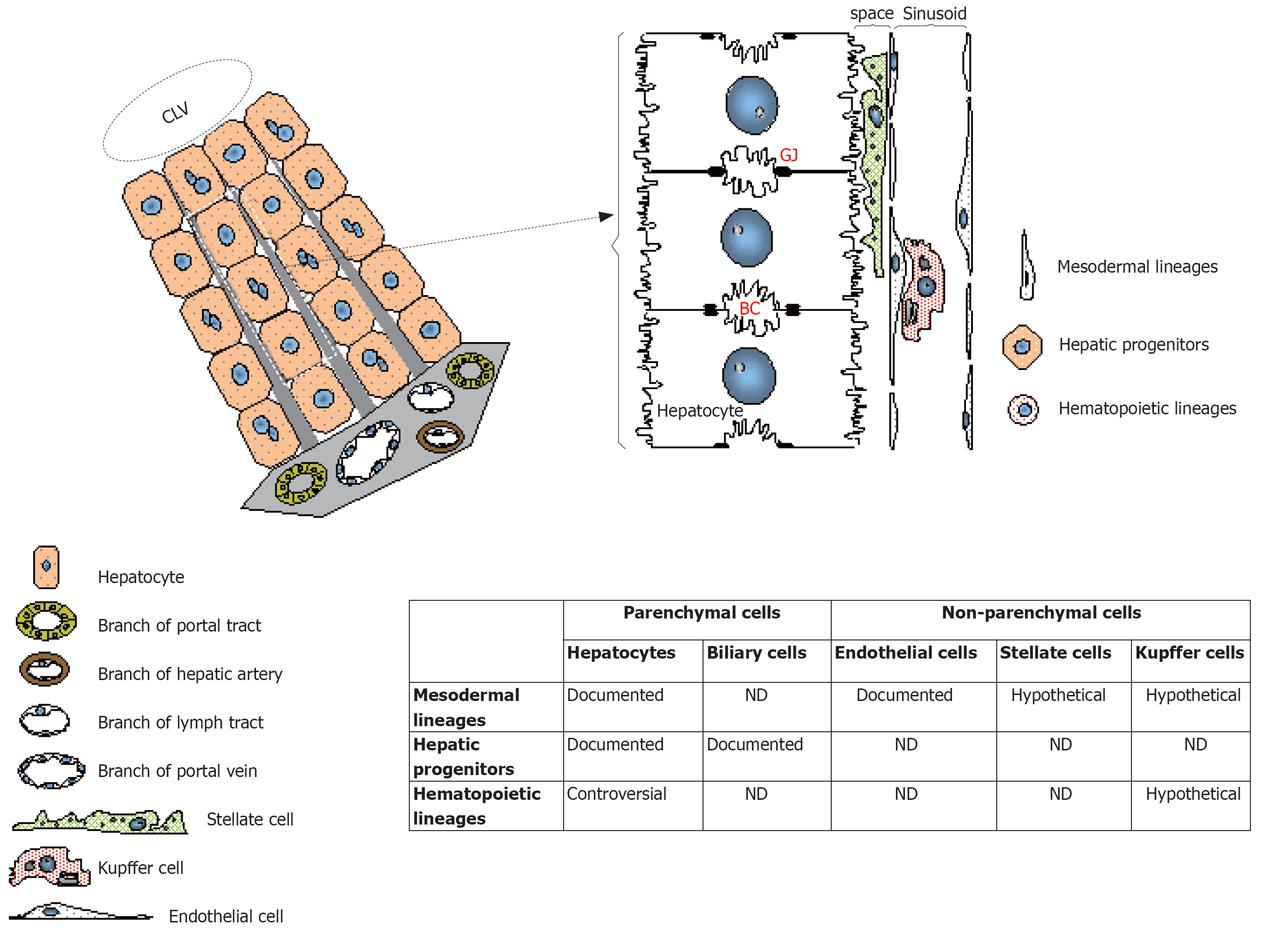
Figure 3 Involvement of stem cells in the regeneration of the liver parenchymal and non-parenchymal cell lineages.
Schema shows the liver architecture and details the topography of the cell populations. In the table are resumed the cell fractions that could potentially be the targets of differentiation after transplantation of stem cells. Some of these differentiation potentials were documented with stem cells while other were contradicted and were thus exposed as controversial. Other differentiation pathways are purely hypothetical based on the embryonic origin of the stem cells. BC: Bile canalicule; CLV: Centrolobular vein; GJ: Gap junctions.
- Citation: Lysy PA, Campard D, Smets F, Najimi M, Sokal EM. Stem cells for liver tissue repair: Current knowledge and perspectives. World J Gastroenterol 2008; 14(6): 864-875
- URL: https://www.wjgnet.com/1007-9327/full/v14/i6/864.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.864