Copyright
©2008 The WJG Press and Baishideng.
World J Gastroenterol. Dec 14, 2008; 14(46): 7033-7058
Published online Dec 14, 2008. doi: 10.3748/wjg.14.7033
Published online Dec 14, 2008. doi: 10.3748/wjg.14.7033
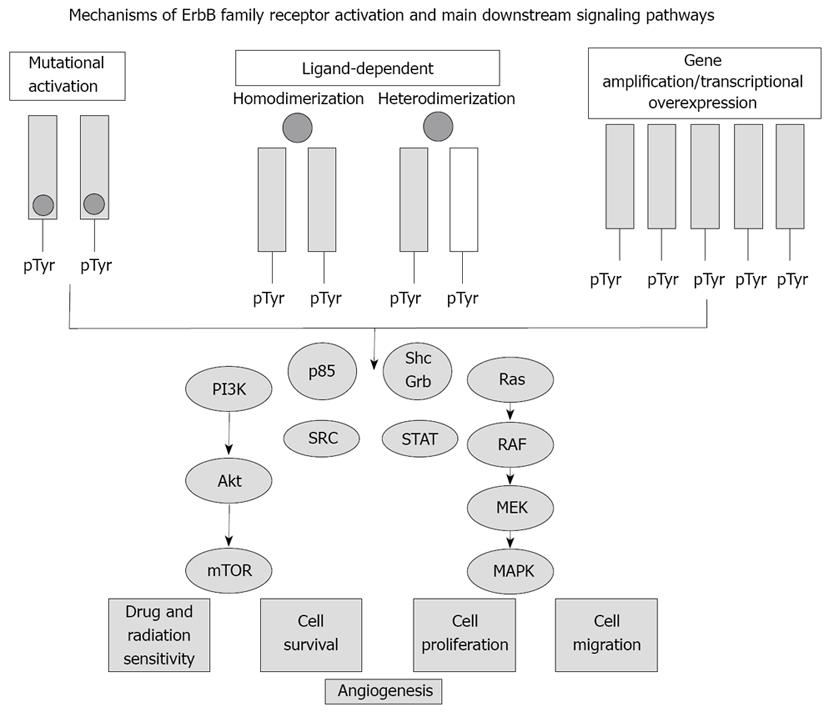
Figure 1 Simplified scheme depicting ErbB receptor tyrosine kinase activation and downstream signaling pathways relevant to intrahepatic cholangiocarcinogenesis.
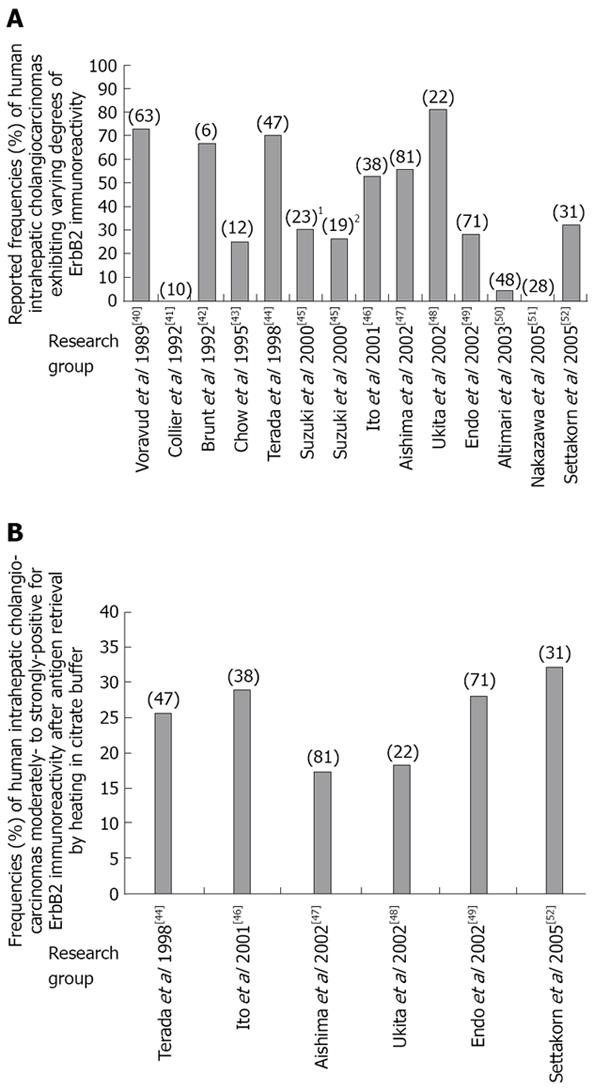
Figure 2 ErbB2 expression in human intrahepatic cholangiocarcinoma.
A: Differences in overall reported frequencies of archival human intrahepatic cholangiocarcinoma specimens scored as being immunohistochemically- positive for ErbB2 expression using non-uniform experimental methods and criteria; B: Range of reported frequencies of archival human intrahepatic cholangiocarcinomas scored as being moderately-to-strongly positive (+2 to +3) for ErbB2 immunoreactivity following antigen unmasking by heating of tissue specimens in citric acid buffer, pH 6.0. Number in () = number of cases analyzed; number in [] = reference number; In A, 1refers to Japanese cases analyzed; 2refers to Thai cases analyzed.
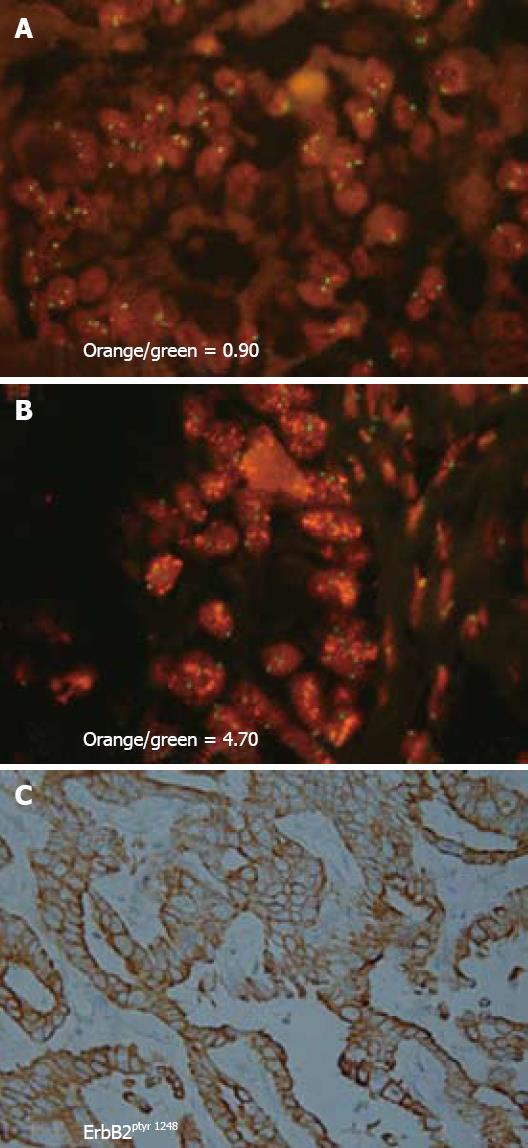
Figure 3 Representative photomicrographs demonstrating c-erbB2 gene amplification and a corresponding strong positive immunoreactivity for activated ErbB2 oncoprotein in the neoplastic cholangiocytes of a human intrahepatic cholangiocarcinoma.
A and B: Sections of two different intrahepatic cholangiocarcinomas with neoplastic cholangiocytes exhibiting FISH nuclear signals for c-erbB2 (orange dots) and for CEP17 (centromeric region of chromosome 17; green dots). Orange/green values indicate c-erbB2/CEP17 signal ratio for each cholangiocarcinoma. An orange/green ratio of > 2.0 = a tumor with c-erbB2 amplification (B), whereas a ratio value at or approximating 1.0 = a tumor without c-erbB2 amplification (A). The neoplastic cholangiocytes in the intrahepatic cholangiocarcinomas depicted in A (immunohistochemical staining not shown) and in B (with corresponding immunohistochemical staining shown in C) were each found to exhibit strong positive immunoreactivity at the plasma membrane for activated ErbB2 oncoprotein, using an antibody directed against phosphotyrosine 1248 of ErbB2. Tyrosine 1248 is the main autophosphorylation site in the carboxy terminal domain of ErbB2 linked to downstream signaling through the ras-raf-MEK-p42/44 MAPK signal transduction pathway. Thus, activation of ErbB2 via autophosphorylation at tyrosine 1248 in neoplastic cholangiocytes of intrahepatic cholangiocarcinomas can be demonstrated either in the presence or absence of c-erbB-2 amplification. (A, B × 330, C × 132).
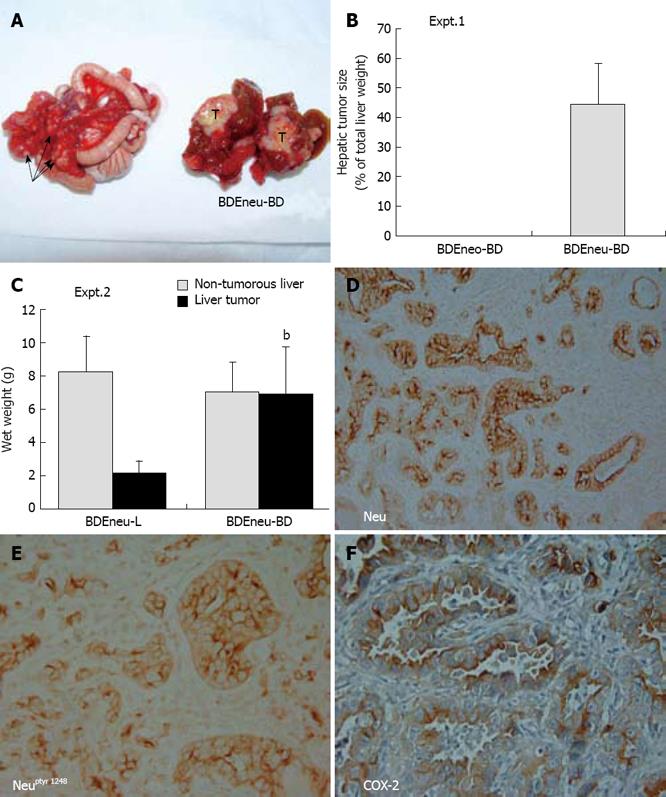
Figure 4 Rat BDEneu cell transplantation model of intrahepatic cholangiocarcinoma progression.
A: Gross pathology of a bisected invasive hepatic tumor (T) formed in the liver of an isogenic Fischer 344 rat at 3 wk after bile duct inoculation of 4 x 106 tumorigenic rat BDEneu cholangiocytes overexpressing mutationally-activated Neu, together with associated peritoneal metastases (arrows); B: Expt 1. mean tumor size ± SD (as a % of total liver weight) of hepatic tumors that formed at a 100% incidence in isogenic rat livers at 3 wk after bile duct inoculation of 4 x 106 BDEneu cells (BDEneu-BD), compared with no tumors formed in rats comparably inoculated with control non-tumorigenic BDEneo cells (BDEneo-BD) transfected to stably express the neomycin resistance gene only, in the absence of oncogenic neu n = 6; C: Expt 2. Differences in mean wet weight ± SD of hepatic tumors (relative to that of corresponding non-tumorous liver) formed at 3 wk after bile duct inoculation of 4 x 106 BDEneu cells (BDEneu-BD) versus the mean wet weight of hepatic tumors that formed over the same time period following inoculation of the BDEneu cells directly into liver (BDEneu-L). bP≤ 0.001 (BDEneu-BD tumor versus BDEneu-L tumor). n = 4; D: Representative low power photomicrograph demonstrating neoplastic cholangiocytes within a rapidly growing BDEneu-BD intrahepatic cholangiocarcinoma to be strongly immunoreactive for plasma membrane Neu; E: Photomicrograph showing malignant cholangiocytes within a BDEneu-BD intrahepatic cholangiocarcinoma to exhibit strong uniformly positive immunoreactivity for activated Neu phosphorylated at tyrosine 1248; F: Photomicrograph of a BDEneu-BD tumor demonstrating positive cytoplasmic immunostaining for COX-2 in the cholangiocytes of the neoplastic ducts. Positive immunostaining in D-F is indicated by brown reaction product. (See References 68 and 69 for a more complete description of rat BDEneu model). (D × 66, E, F × 132).
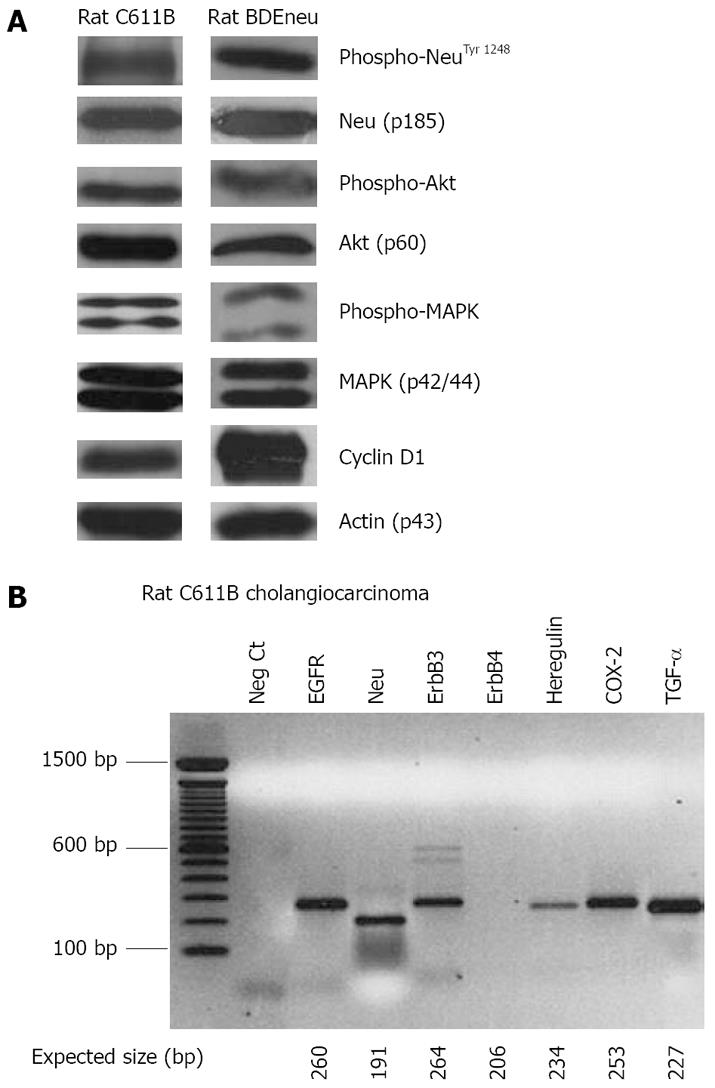
Figure 5 Western blot (A) and RT-PCR (B) analyses.
A: Relevant protein and phosphorylation profiles associated with aberrant Neu expression in rat C611B and BDEneu cholangiocarcinoma cell lines. C611B overexpress the wild-type c-neu gene[64], whereas BDEneu cells overexpress mutationally-activated neu oncogene[68,69]. Note that both cell lines are positive for phospho-Neu, phospho-Akt, and phospho-p42/44 MAPK, as well as show prominent expression of cyclin D1; B: RT-PCR analysis of c-erbB family receptor together with heregulin, TGF-α, and COX-2 mRNAs detected in malignant neoplastic ducts obtained by laser capture microdissection from a hepatic tumor formed in an isogenic Fischer 344 male rat following cell transplantation of rat C611B cholangiocarcinoma cells directly into liver. Note that as expected, among the ErbB family receptors, only ErbB4 mRNA is not detected in the tumor ducts. Moreover, it is also significant that mRNA signals for both the EGFR ligand TGF-α and for COX-2, which is known to be up-regulated in response to EGFR and/or Neu signaling, are each shown to be prominently detected in C611B cholangiocarcinoma.
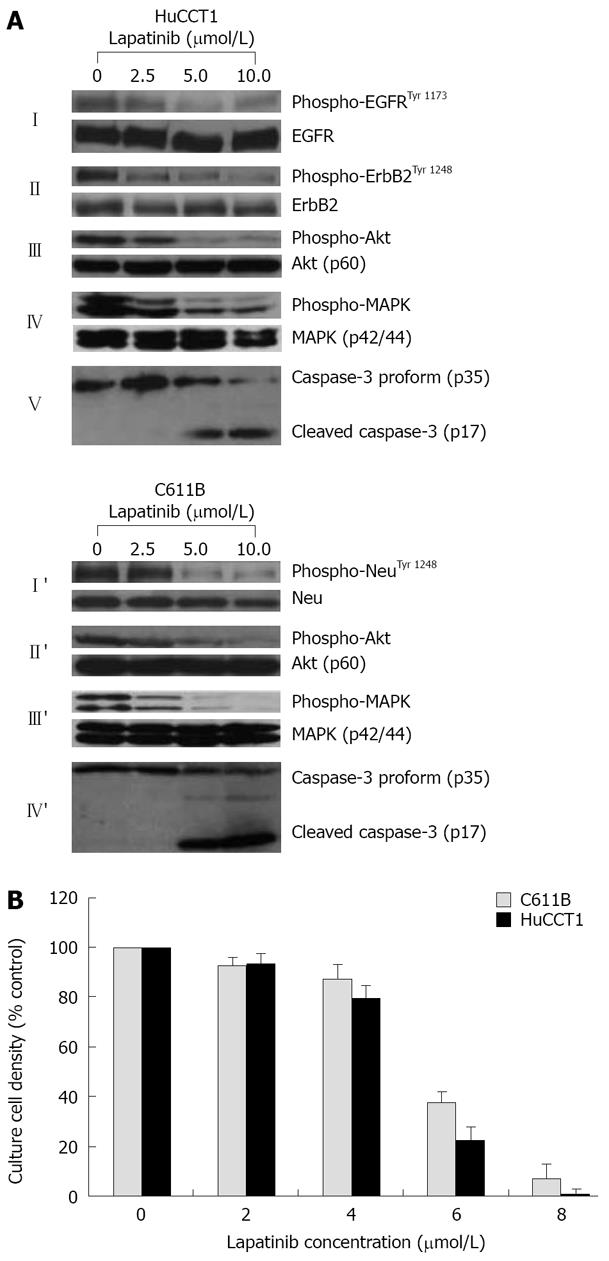
Figure 6 Dose-dependent inhibition test.
A: Western blots demonstrating dose-dependent suppression by the dual ErbB1 (EGFR)/ErbB2 (Neu) inhibitor lapatinib of phospho-EGFRTyr 1173 and/or of phospho-ErbB2/NeuTyr 1248, together with concomitant downstream inhibition of phospho-Akt and of phospho-p42/44 MAPK expressed in cultured human HuCCT1 and in rat C611B cholangiocarcinoma cells, respectively. Note that the lapatinib treatment in vitro did not affect corresponding total protein levels, but in both cell lines induced a dose-dependent activation of the apoptotic enzyme, caspase-3; B: Dose response curves for lapatinib on suppressing anchorage-dependent growth of cultured rat C611B and human HuCCT1 cholangiocarcinoma cells. Each value represents the mean ± SD (n = 3). Note the close relationship between the lapatinib concentrations required to induce prominent suppression of EGFR and/or ErbB-2/Neu signaling (≥ 5 μmol/L) and those associated with marked inhibition of in vitro cholangiocarcinoma cell growth (≥ 6 μmol/L). Lapatinib-induced suppression of C611B and HuCCT1 growth in vitro correlated with both a down-regulation of cyclin D1 protein expression and with a prominent apoptosis being induced in these respective cultured cholangiocarcinoma cell lines (data not shown).
- Citation: Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol 2008; 14(46): 7033-7058
- URL: https://www.wjgnet.com/1007-9327/full/v14/i46/7033.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7033