Copyright
©2008 The WJG Press and Baishideng.
World J Gastroenterol. Nov 28, 2008; 14(44): 6786-6801
Published online Nov 28, 2008. doi: 10.3748/wjg.14.6786
Published online Nov 28, 2008. doi: 10.3748/wjg.14.6786
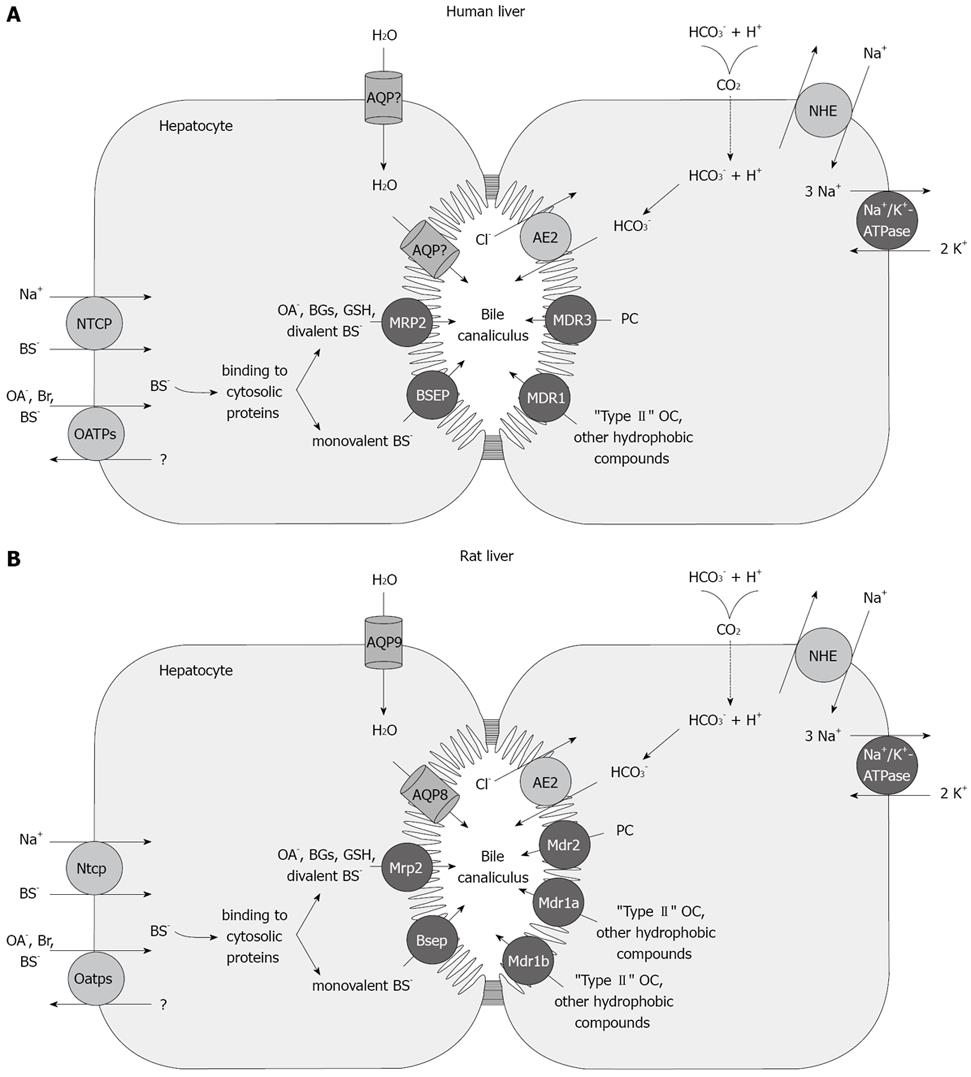
Figure 1 Localization and function of sinusoidal and canalicular hepatocellular transporters.
A: humans; B: rodents. The Na+-dependent sinusoidal uptake of bile salts is mediated by NTCP (human)/Ntcp (rat). The Na+-independent hepatic uptake of organic anions (OA-), Bile salts and type II organic cations (OC+) is mediated by members of the OATP/Oatp family. Sinusoidal uptake of type I OC+ is mediated by OCT1/Oct1. Transport across the canalicular membrane is driven mainly by ATP-dependent export pumps (black circles). MDR1/Mdr1a, Mdr1b mediates canalicular excretion of amphiphilic type II OC+ and other hydrophobic compounds. MDR3/Mdr2 functions as a phosphatidylcholine (PC) flippase. BSEP/Bsep mediates apical excretion of BSs. MRP2/Mrp2 transports non-bile-salt organic anions, such as bilirubin glucuronides, GSH, and sulfated/glucuronidated bile salts. Canalicular transport of HCO3- is mediated by the Cl-/HCO3- exchanger AE2/Ae2. Aquaporins AQP9 and AQP8 are involved in the transport of water across the rat sinusoidal and the canalicular membrane, respectively. The nature of the water channels in human liver has yet to be characterized.
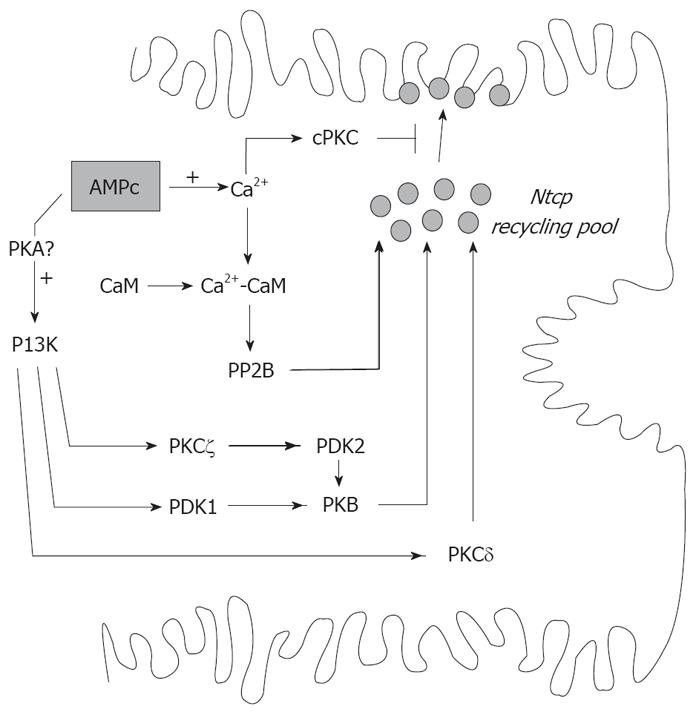
Figure 2 Signaling pathways that regulate the cAMP-induced exocytic insertion of Ntcp into the basolateral membrane.
cAMP stimulatory effect involves elevations in cytosolic Ca2+ and activation of PI3K-dependent pathway, probably via protein kinase A (PKA). CaM complex activates phosphatase 2B (PP2B), which promotes insertion of Ntcp by dephosphorylation. This pathway is counter-regulated by cPKC. cAMP also stimulates Ntcp targeting by PI3K-dependent activation of PDK1 and subsequent PKB activation. Alternatively, PKB is activated by the concerted action of the atypical PKCζ and PDK2. Finally, cAMP/PI3K signaling stimulatory pathway may involve PKCδ.
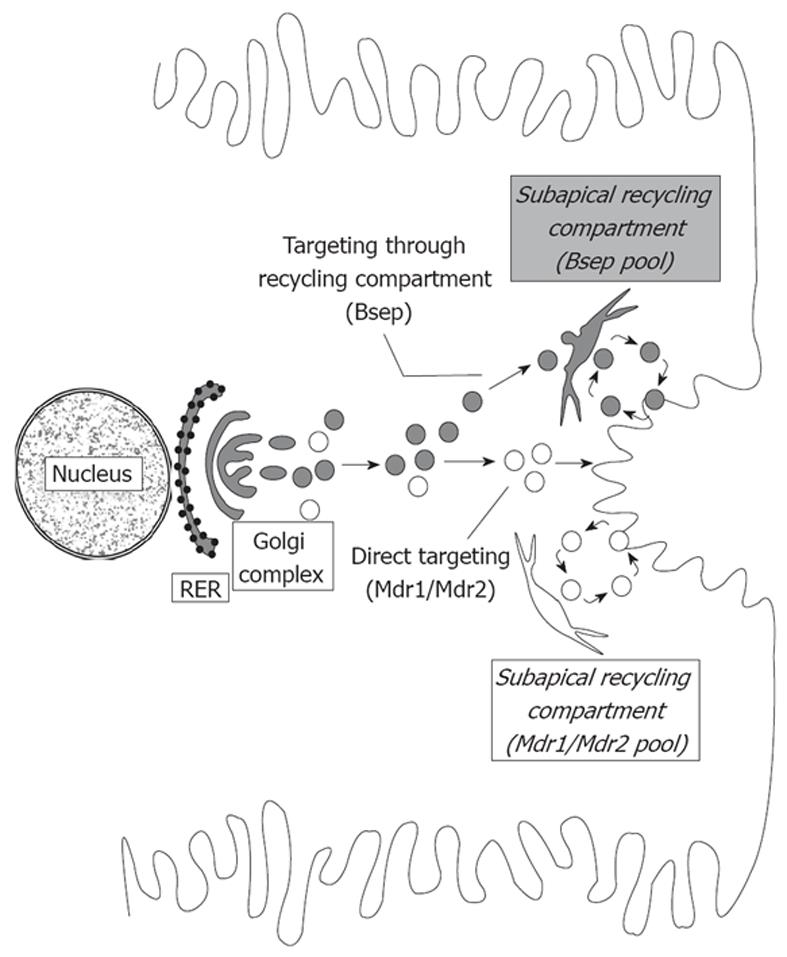
Figure 3 Routes involved in trafficking of canalicular transporters.
The trafficking of vesicles delivering Bsep (gray vesicles) or Mdr1/Mdr2 (white vesicles) from the site of synthesis to the canalicular domain is distinct. Mdr1 and Mdr2 are directly targeted to the canalicular membrane, whereas Bsep is indirectly targeted via a subapical, endosomal compartment, which allows the recycling of transporters (exocytic insertion/endocytic internalization). Once targeted, Mdr1 and Mdr2 are also able to recycle between the subapical compartment and the canalicular membrane.
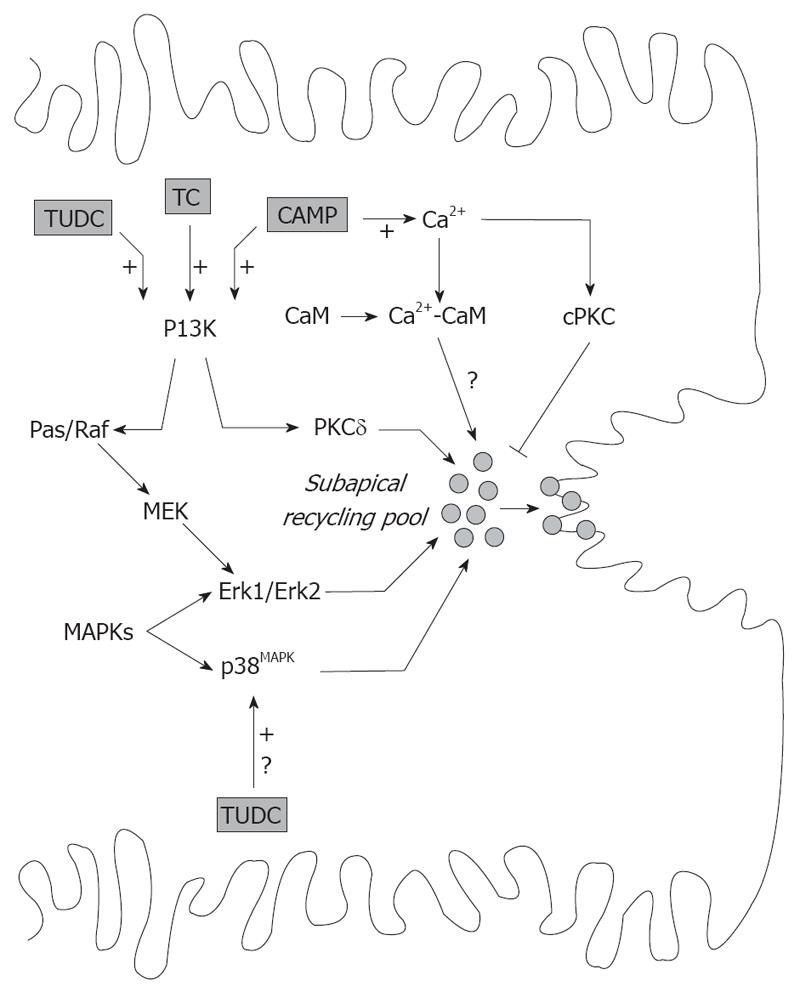
Figure 4 Signaling pathways involved in the exocytic insertion of canalicular transporters promoted by cAMP and by TC and TUDC.
cAMP effect involves elevation in cytosolic Ca2+ and activation of the PI3K-dependent pathway. Formation of the CaM complex promotes apical insertion of transporters via unidentified mediators, and is counter-regulated by activation of cPKC. PI3K promotes exocytic insertion of canalicular transporters by activation of PKCδ and Erk-1 and Erk-2 of MAPK, via the Ras/Raf- MAPK kinase (MEK)-Erk-1/2 pathway. TC and TUDC also evoke the PI3K-dependent signaling pathway and promote insertion of canalicular transporters via the Ras/Raf-MEK-Erk-1/2 pathway. TUDC also stimulates canalicular carrier insertion by activation of MAPKs of the p38MAPK type, by an unknown mechanism.
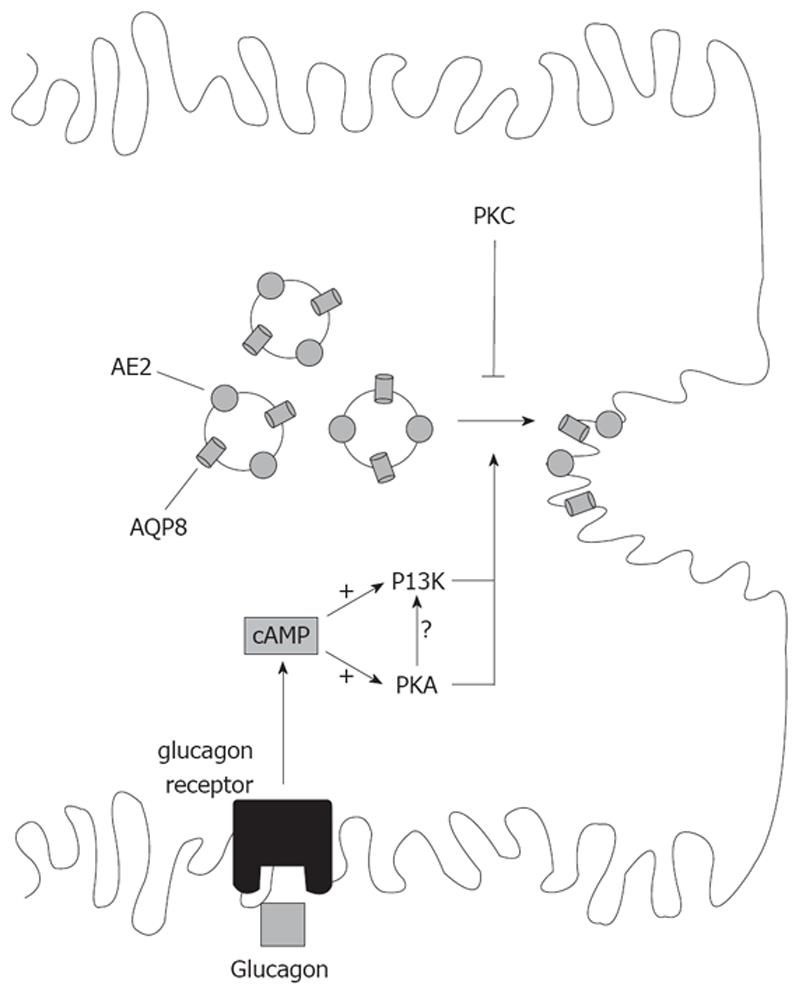
Figure 5 Signaling pathways involved in the co-stimulation of the canalicular targeting of AE2 and AQP8 by cAMP.
AE2 and AQP8 are co-localized in the same population of pericanalicular vesicles, thus explaining common signaling modulation. cAMP stimulates AE2 and AQP8 targeting via activation of PKA. The PI3K pathway mediates the cAMP-stimulated, PKA-dependent targeting of AQP8, and probably that of AE2. cAMP effect on both transporters is counteracted by activation of PKC.
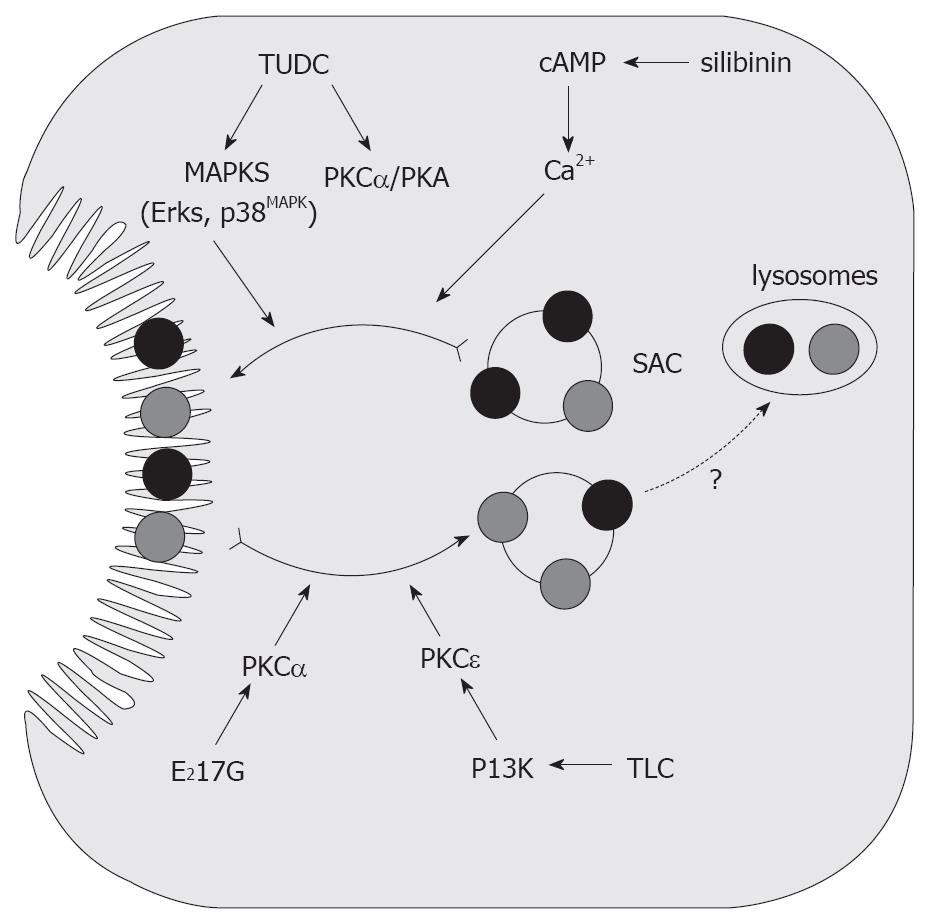
Figure 6 Endocytic internalization of canalicular transporters in E217G and in TLC-induced cholestasis.
Protection from these cholestatic agents by the anticholestatic agents cAMP and TUDC is also shown. E217G and TLC induce endocytic internalization of canalicular transporters into the subapical compartment (SAC); this may lead to delivery to the lysosomal compartment, followed by degradation. E217G-induced activation of PKCα and TLC-induced, phosphatidylinositol 3-kinase (PI3K)-dependent activation of PKCε have been proposed to mediate this retrieval. Elevation of intracellular cAMP levels induced by administration of the permeant cAMP analogue DBcAMP, or by the phosphodiesterase inhibitor silibinin, prevents internalization, and accelerates re-insertion, via cytosolic Ca2+ elevations. On the other hand, TUDC prevents transporter endocytosis probably via co-stimulation of PKCα- and PKA-dependent pathways.
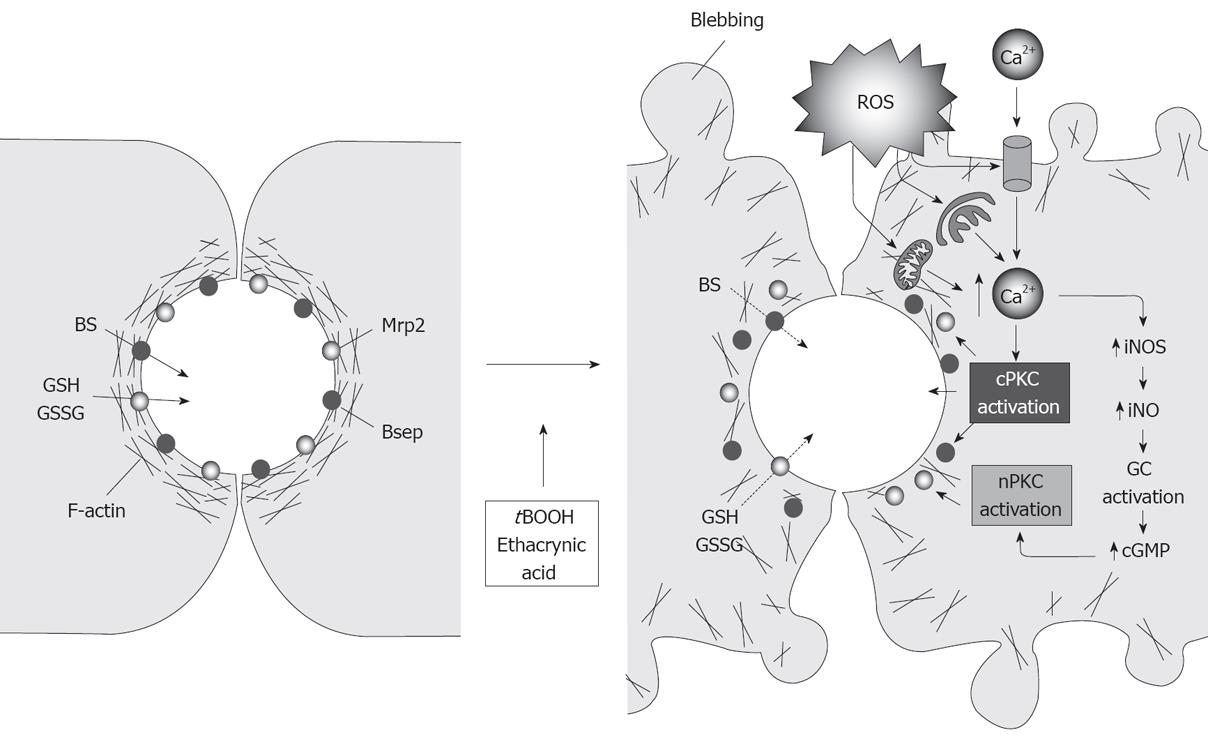
Figure 7 Endocytic internalization of canalicular transporters under oxidative stress.
In normal cells, the pericanalicular arrangement of F-actin allows for the appropriate insertion of the canalicular transporters in their membrane domain. Reactive oxygen species produced by the administration of oxidizing compounds, such as tBOOH or ethacrynic acid, induces mobilization of Ca2+ across the plasma membrane and membranes of the calciosome (smooth ER and mitochondria), and the subsequent activation of cPKC. cPKC activation induces blebbing and redistribution of F-actin from the pericanalicular region to the cell body. This rearrangement, in turn, leads to canalicular transporter internalization. Moderate Ca2+ elevations may also activate iNOS, which induces NO-mediated guanylate cyclase activation and further cGMP-mediated activation of nPKC, which may internalize selectively Mrp2.
- Citation: Roma MG, Crocenzi FA, Mottino AD. Dynamic localization of hepatocellular transporters in health and disease. World J Gastroenterol 2008; 14(44): 6786-6801
- URL: https://www.wjgnet.com/1007-9327/full/v14/i44/6786.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6786