Copyright
©2008 The WJG Press and Baishideng.
World J Gastroenterol. Jan 21, 2008; 14(3): 390-400
Published online Jan 21, 2008. doi: 10.3748/wjg.14.390
Published online Jan 21, 2008. doi: 10.3748/wjg.14.390
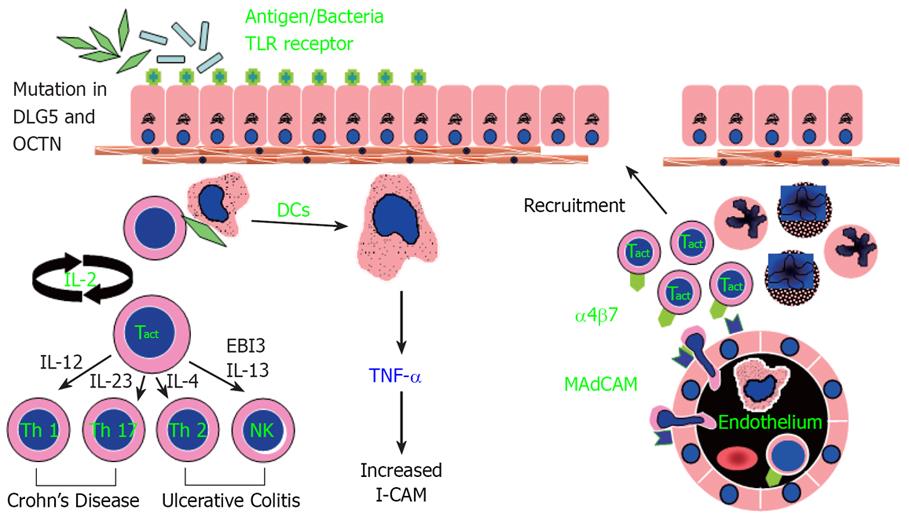
Figure 1 Working hypothesis of inflammatory bowel disease.
A defect occurs in sampling of gut luminal antigen, possibly mediated by enhanced Toll-like receptor activity and controlled by other genetic factors (mutations in DLG5 and OCTN). Over-response to antigens results in stimulated dendritic cells (DC) that recruits and generates various T-cell subtypes, which then initiate a cascade of immunologic events leading to mucosal inflammation. Adhesion molecules such as intercellular cell adhesion molecule 1 (ICAM1) are important for circulating mononuclear and polymorphonuclear cells to adhere and migrate to the inflamed gut mucosa. Crohn’s disease (CD) is a predominately Th1 and Th17 mediated process, while ulcerative colitis (UC) appears to be predominately mediated through Th2 and NK T-cells.
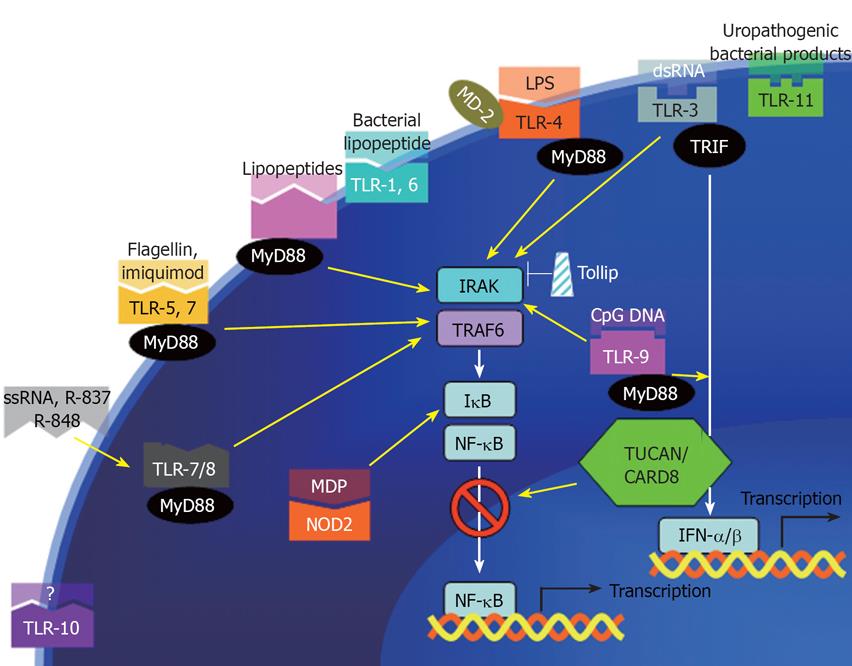
Figure 2 PRRs and their corresponding ligands.
Toll-like receptors on the cell membrane (TLR-1, -6, -10, and -11) and intracellular (TLR-7, -8, and -9) selectively bind to various bacterial, viral, or fungal components. A major convergent pathway is through myeloid differentiation primary response protein MyD88, which activates NF-κB. The death domain of MyD88 then recruits downstream IL-1 receptor-associated kinase (IRAK) to the receptor complex. IRAK is then autophosphorylated and in turn recruits TNF receptor-associated factor 6 (TRAF6). TRAF6 then activates kinases including NF-κB-inducing kinase (NIK) and mitogen-activated protein kinase/ERK kinase kinase 1 (MEKK1). Inhibitor of NF-κB degradation (IκB) is subsequently phosphorylated and degraded, resulting in NF-κB nuclear translocation. NF-κB then activate genes involved in inflammatory response including IL-1b, TNF, IL-6, IL-8, and ICAM1. Toll-inhibitory protein (Tollip) is one of the negative regulators of the innate immunity. Activation of typeI IFN (IFN-α/β) also has anti-inflammatory function in colitis.
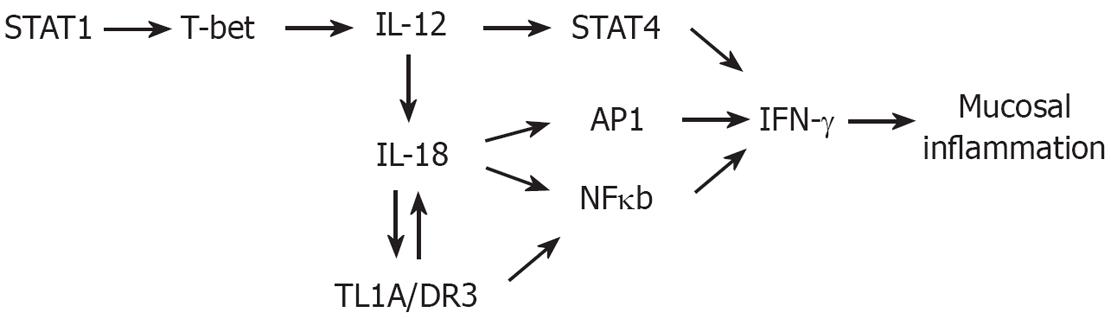
Figure 3 Th1 Polarization Pathway.
Polarization of naïve T-cells towards Th1 cell subtype is initially induced by IL-12, a heterodimer of the p40 and p35 subunits. Activation of signal transducer and activator of transcription-1 (STAT1) and its stimulation of transcription factor T-bet, a TH1 “master switch” that upregulates and stabilizes the expression of IL-12. IL-12 can then amplify TH1 response by upregulating IL-18 on T-cells. IL-18 then stimulates NF-κB and AP1, and in synergy with STAT4 (activated by IL-12), transactivates IFN-γ expression.
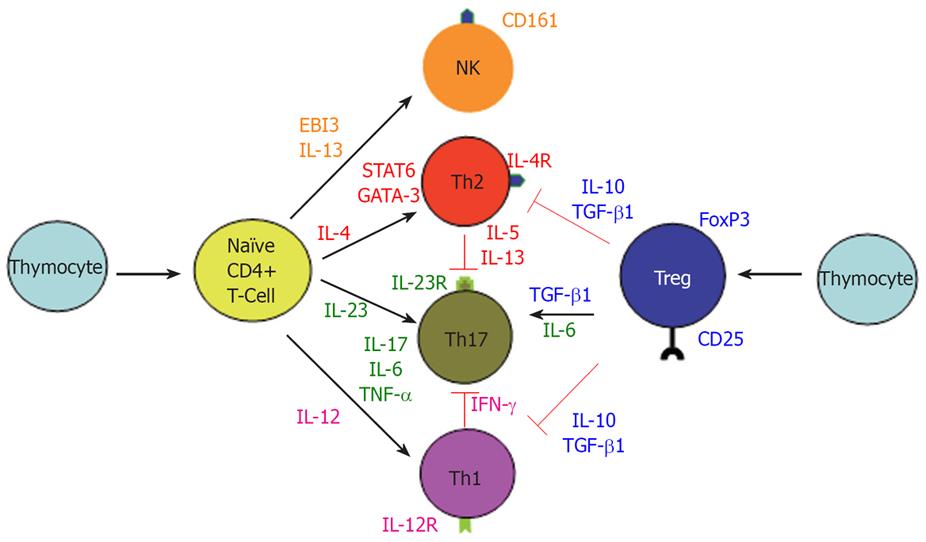
Figure 4 Differentiation of T-cell Subsets.
Upon stimulation, naïve CD4+ T-cells differentiate into 3 main subsets, Th1, Th2, and Th17 cell. IL-12 induces the formation of IFN-γ producing Th1 cells. IL-23 promotes the development of an IL-17 producing CD4+ helper T cells. IL-4 induces STAT6 activation, promoting the expression of GATA-3, which feed forward to induce IL-4 expression and Th2 cell differentiation. An EBI3-associated cytokine was hypothesized to be necessary for activation of IL-13 producing NK T-cells. Tregs, an immune-modulating subset of CD4+ T-cells, can suppress the differentiation and function of Th1 and Th2 cells. Interestingly, in the presence of IL-6, Treg-derived TGF-β can induce the differentiation of Th17 cells.
- Citation: Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol 2008; 14(3): 390-400
- URL: https://www.wjgnet.com/1007-9327/full/v14/i3/390.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.390