Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 28, 2007; 13(4): 532-537
Published online Jan 28, 2007. doi: 10.3748/wjg.v13.i4.532
Published online Jan 28, 2007. doi: 10.3748/wjg.v13.i4.532
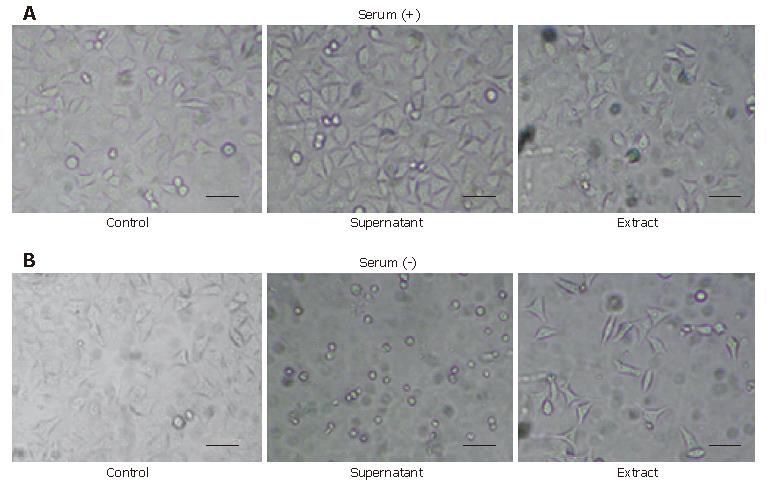
Figure 1 Morphologic change of HeLa cells caused by the supernatant and the extract of H pylori with or without serum after 24 h.
HeLa cells were seeded at 5 000 cells per well in every plate. The concentration of the supernatant cultured with or without serum was 2.1 mg/mL and the concentration of the respective extract was 0.5 mg/mL. A: HeLa cells exposed to the supernatant of the method using serum showed little change and the ones exposed to the extract also showed no remarkable changes but included a few minor vacuolations compared to the control; B: HeLa cells exposed to the supernatant of the method without serum showed a round-formed shape and a decayed contour with greatly decreased cell number, and those exposed to its extract showed an elongated or spindle-like shape with a decrease in cell number. Scale bar, 200 μm.
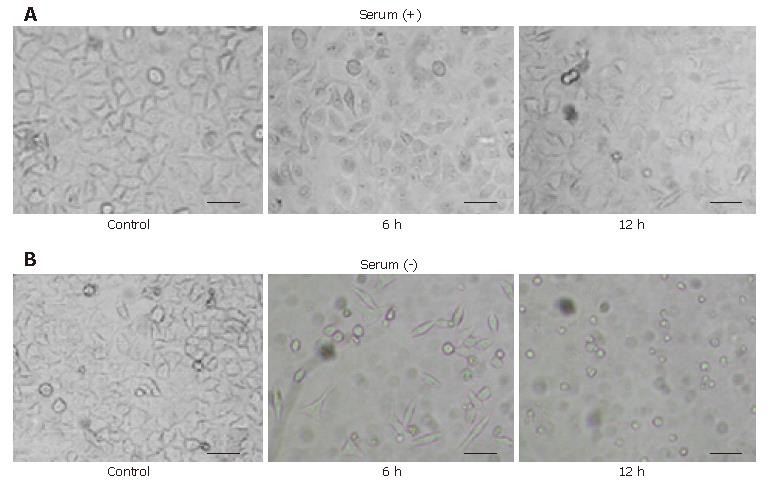
Figure 2 Morphologic change of HeLa cells caused by H pylori 1.
0 mg/mL cell extract with or without serum. HeLa cells exposed to the extract cultured without serum showed the characteristic morphological changes in 12 h. A: HeLa cells exposed to the extract cultured with serum 6 h and 12 h after addition of the extract showed no remarkable changes but a small decrease in cell number; B: HeLa cells exposed to the extract cultured without serum 6 h and 12 h after addition of the extract showed the round-formed shape or round debris with a decayed contour through the elongated or spindle-like shape without vacuolations. Scale bar, 200 μm.
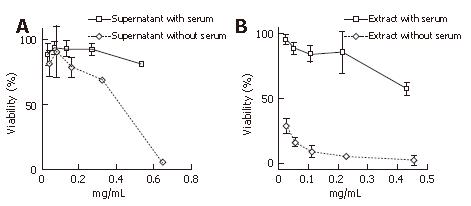
Figure 3 Cell viability assay of the supernatant and the extract of serum-free cultured H pylori at the onset of culturing and long cultured H pylori, approximately 100 passages after the onset of culturing.
A: Long serum-free cultured supernatant showing dose-dependent decrease curves of cell viability; B: Long serum-free cultured extract showing further dose-dependent decrease curves of cell viability. Each point represents the mean ± SD from three separate experiments.
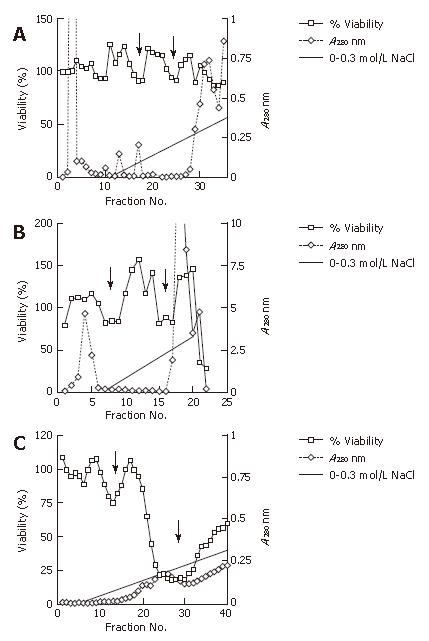
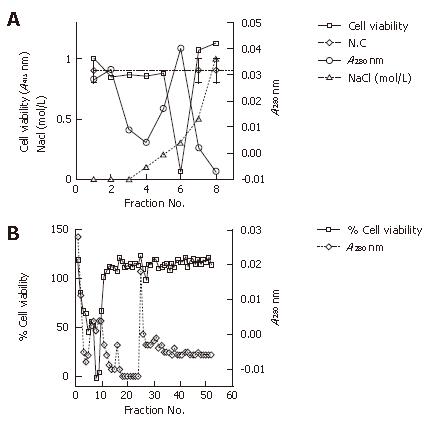
Figure 4 Analysis of the column anion exchange chromatography of the extract cultured without serum.
Cell viability that represents the putative cytotoxic factor was measured for cell viability at A415 using the WST1 method. Column eluates that represent the protein densities were monitored at A280 for each of the filtrated fractions. Proteins were eluted with a linear gradient of 0-0.3 mol/L NaCl. A: Anion exchange chromatography of the cell extract protein at the onset of culturing without serum. Two minor dips of cell viability are indicated (arrows); B: Anion exchange chromatography of the cell extract protein approximately 50 passages (approximately 6 mo) after the onset of culturing without serum. Two clearer minor dips of cell viabilities are indicated (arrows); C: Anion exchange chromatography of the cell extract protein approximately 100 passages (approximately one year) after the onset of culturing without serum. One minor dip and one major dip are at 0.1 mol/L NaCl and 0.2 mol/L NaCl, respectively (arrows).
Figure 5 Analysis of the extract cultured without serum.
Cell viability that represents the putative cytotoxic factor was measured for cell viability at A415 using the WST1 method. Column eluates that represent protein densities were monitored at A280 for each filtrated fraction. A: Cation exchange chromatography of the cell extract protein approximately 100 passages (approximately one year) after the onset of culturing without serum. The cell extract proteins were eluted with a stepwise gradient of 0-0.5 mol/L NaCl; B: Size exclusion chromatography of the cell extract protein approximately 100 passages (approximately one year) after the onset of culturing without serum.
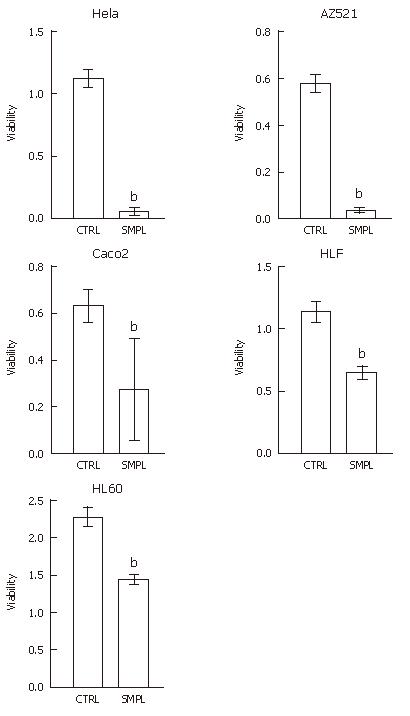
Figure 6 Cytotoxicity of the extract partially purified by anion exchange chromatography.
Each bar represents mean + SE of three separate experiments. CTRL: control lysate; SMPL: sample lysate. bP < 0.01 vs CTRL.
-
Citation: Ohno H, Murano A. Serum-free culture of
H pylori intensifies cytotoxicity. World J Gastroenterol 2007; 13(4): 532-537 - URL: https://www.wjgnet.com/1007-9327/full/v13/i4/532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i4.532