Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 21, 2007; 13(35): 4716-4724
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4716
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4716
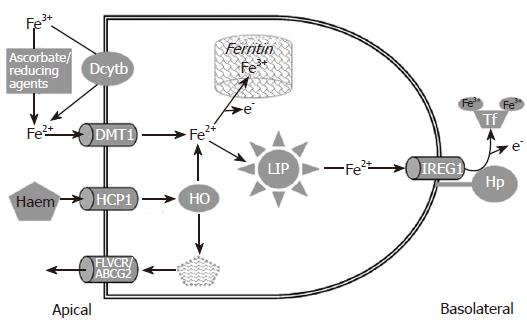
Figure 1 The cellular mechanisms involved in intestinal iron absorption.
Dietary non-haem iron (mostly ferric) is reduced by the actions of the ferric reductase Dcytb and reducing agents in the diet to yield Fe2+, which subsequently enters the enterocytes via DMT1. Haem is absorbed via HCP1, broken down by haem oxygenase 1 (HO) to liberate Fe2+ (this joins a common pool with iron from the non-haem pathway) and bilirubin (which might be removed from the cell by the efflux proteins FLVCR and ABCG2). If body iron stores are high, iron may be diverted into ferritin and lost when the cell is shed at the villus tip. Alternatively, iron passes into the labile iron pool (LIP) and is subsequently processed for efflux via IREG1 (as Fe2+). The exiting iron is re-oxidised to Fe3+ through hephaestin (Hp) to enable loading onto transferrin (Tf).
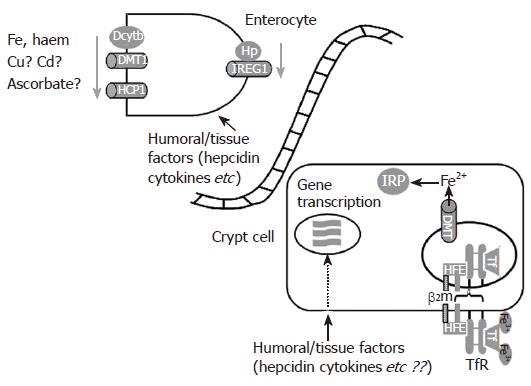
Figure 2 The regulation of intestinal iron transport.
The duodenal crypt-villus axis represents a differentiation pathway that can be influenced by the dietary and humoral signals that ultimately regulate iron absorption. Immature proliferating cells in the crypt take up transferrin from the circulation via transferrin receptors (TfR). This process is governed by interactions between Hfe, β2microglobulin and TfR. The crypt cells are thus sensitive to the circulating levels and the iron-saturation of Tf. Iron regulatory proteins (IRP) recognise the cellular iron content and regulate iron responsive mRNAs posttranscriptionally. In addition, transcription control of iron metabolism may be exerted by a number of humoral and tissue-derived factors, such as hepcidin or cytokines interacting with their individual specific receptors. Together, these processes set the basal level of iron transporter protein expression in enterocytes as they mature and travel up the villus. Importantly, this basal level of transporter expression can be modified rapidly in mature enterocytes in response to changes in the levels of dietary factors (e.g. the iron content of the diet, other metals etc) and humoral and local tissue mediators (especially hepcidin and pro-inflammatory cytokines). This combination of basal programming in the crypts and fine tuning in villus enterocytes permits tight control of intestinal iron transport.
- Citation: Sharp P, Srai SK. Molecular mechanisms involved in intestinal iron absorption. World J Gastroenterol 2007; 13(35): 4716-4724
- URL: https://www.wjgnet.com/1007-9327/full/v13/i35/4716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i35.4716