Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 21, 2007; 13(35): 4673-4689
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4673
Published online Sep 21, 2007. doi: 10.3748/wjg.v13.i35.4673
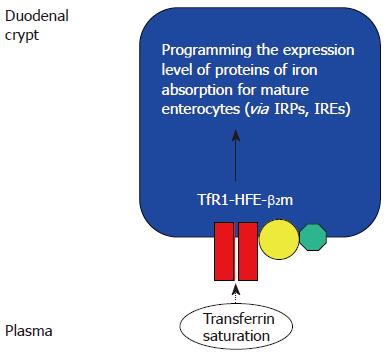
Figure 1 The duodenal “crypt cell hypothesis” of HFE function[36].
HFE at the basolateral membrane of duodenal crypt cells co-localizes with β2-microglobulin and TfR1. The saturation of circulating transferrin, which reflects body iron stores, was proposed to be “sensed” by the TfR1-HFE-β2-microglobulin complex, through transferrin-mediated endocytosis. Wild type HFE was proposed to facilitate transferrin-mediated iron uptake. In haemochromatosis, deficiency of functional HFE would therefore decrease the iron pool within the crypt cell, despite increased body iron stores. This would increase the activity of iron responsive proteins (IRPs), leading to increased expression of genes involved in iron absorption, such as DMT1 and ferroportin. This could contribute to the iron overload seen in HH[27,34,35]. However, recent evidence suggests that HFE may play more important roles in influencing iron metabolism in the liver.
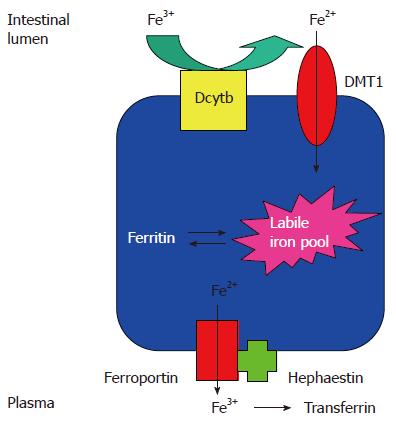
Figure 2 The pathway of iron absorption in the mature duodenal enterocyte.
Dietary ferric iron is reduced to the ferrous state by ferric reductase(s), for example duodenal cytochrome b (Dcytb), which is expressed on the luminal surface of the enterocytes. Ferrous iron is taken up via DMT1 into the labile iron pool. Iron may be stored within the cell as ferritin or transferred across the basolateral membrane to the plasma by ferroportin. The exported iron is oxidised to the ferric state by hephaestin; ferric iron is then avidly bound by circulating transferrin[39].
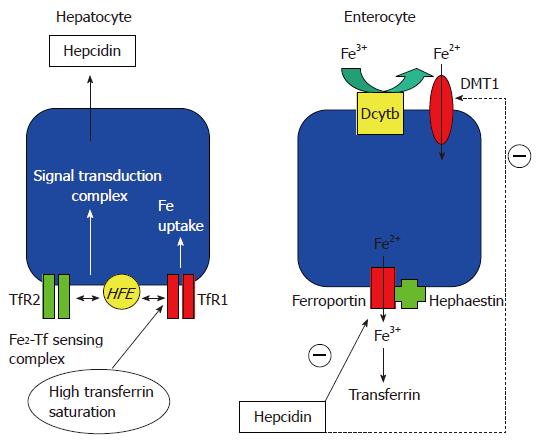
Figure 3 Current concepts regarding hepatic regulation of iron metabolism.
Left panel, hepatocyte. At normal transferrin saturation, TfR1 may sequester HFE[185]. At increased transferrin saturation, diferric transferrin competes with HFE for binding to TfR1[55]. Freed HFE is proposed to bind TfR2; the complex conveys transferrin saturation status via a cytoplasmic signal transduction complex, leading to synthesis and secretion of hepcidin. In HH, mutations in the genes encoding HFE, TfR2, haemojuvelin or hepcidin may all disrupt this sensing system, leading to deficient hepcidin production and iron overload[53]. Right panel, enterocyte. Circulating hepcidin may reduce iron absorption by interacting with ferroportin, causing its internalization and degradation[49] and/or by reducing DMT1 expression[50].
-
Citation: Sebastiani G, Walker AP.
HFE gene in primary and secondary hepatic iron overload. World J Gastroenterol 2007; 13(35): 4673-4689 - URL: https://www.wjgnet.com/1007-9327/full/v13/i35/4673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i35.4673