Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jun 14, 2007; 13(22): 3047-3055
Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3047
Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3047
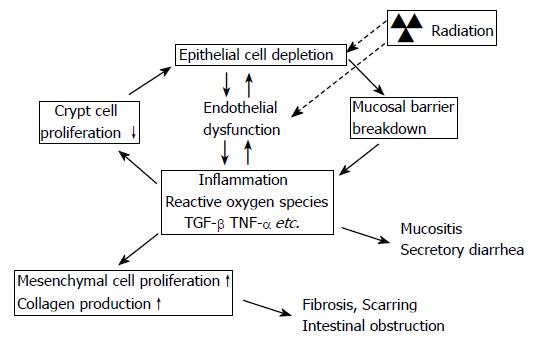
Figure 1 Model of interaction between epithelial and endothelial radiation injury in the intestine demonstrating how endothelial dysfunction may exacerbate the early intestinal radiation response and “drive” the cycle of chronicity of intestinal radiation fibrosis.
Radiation causes epithelial crypt cell death, leading to insufficient replacement of the villus epithelium, and breakdown of the epithelial barrier that normally separates intestinal tissue from the intraluminal contents of the intestine. Simultaneously, radiation causes endothelial dysfunction, notably loss of thromboresistance and increased expression of chemokines and adhesion molecules. The combination of loss of epithelial barrier function and endothelial dysfunction enhances the post-radiation inflammatory response, inhibits restitution of the epithelium, and promotes extracellular matrix deposition.
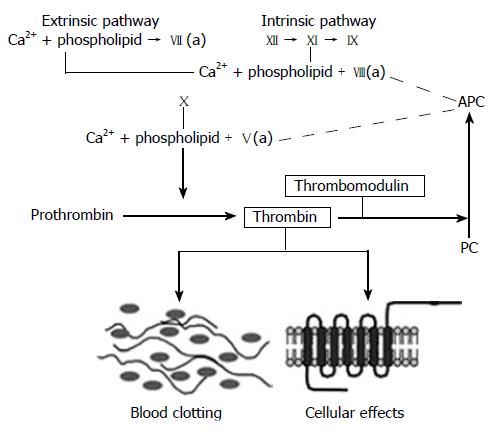
Figure 2 The coagulation cascade.
Simplified diagram of the coagulation “cascade” with the intrinsic, extrinsic, and common pathways. Note how thrombomodulin, located on the luminal surface of endothelial cells, forms a complex with thrombin, which is converted from a pro-coagulant to an anticoagulant and how activated protein C (APC) limits thrombin generation by feed-back into the intrinsic and common coagulation pathways. See text for further details.
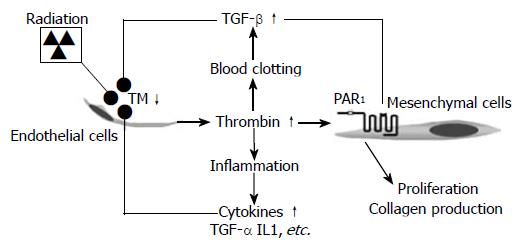
Figure 3 Proposed model linking radiation-induced endothelial dysfunction to chronic inflammation and progressive intestinal fibrosis via chronic PAR1 activation.
Radiation causes TM deficiency in endothelial cells, leading to insufficient “scavenging” of locally formed thrombin. Thrombin exerts pro-coagulant, pro-inflammatory, mitogenic, and pro-fibrogenic effects on mesenchymal cells (smooth muscle cells, fibroblasts, and myofibroblasts), as well as other cell types in the irradiated tissue. Feed-back by cytokines and other inflammatory mediators sustains the endothelial TM deficiency and thus contributes to the chronicity of radiation injury.
- Citation: Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol 2007; 13(22): 3047-3055
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3047.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3047