Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 14, 2007; 13(2): 175-191
Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.175
Published online Jan 14, 2007. doi: 10.3748/wjg.v13.i2.175
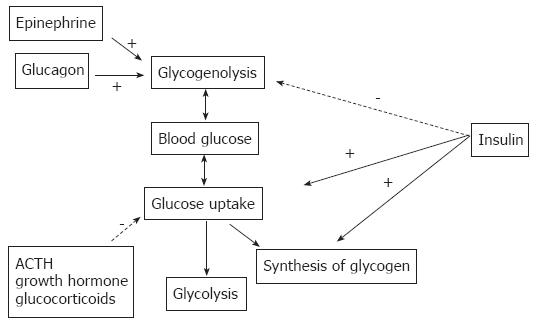
Figure 1 Hormonal regulation of systemic blood glucose.
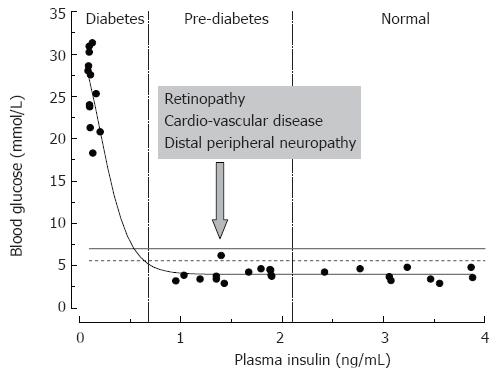
Figure 2 Relationships between rat plasma insulin and fasting blood glucose concentrations.
Data are from normal and streptozotocin-injected adult Sprague-Dawley rats (the authors’ unpublished observations). STZ-rats having moderate pancreatic impairment and moderately decreased plasma insulin (vertical dashed lines) do not develop overt hyperglycemia.
Figure 3 Signs and symptoms of distal peripheral neuropathy.
Categories of symptoms most frequently manifested in humans with diabetes are given in bold.
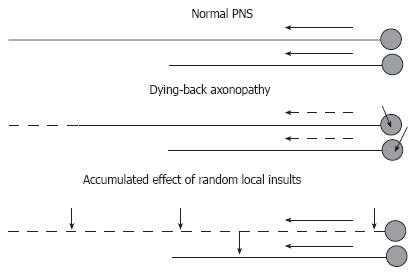
Figure 4 Hypotheses on distal-to-proximal progression of DPN.
In normal PNS (top), neurons synthesize proteins in the cell body and transport them down the axon at the rate determined by axonal structural and functional needs. Impairment of synthesis or axonal transport of proteins will result in dying-back neuropathy, in which neurons with longest processes are affected first (middle). Alternatively, the neuropathy may result from effect of random local insults to the axon, with probability of accumulation of a critical number of such insults being higher for neurons having longer axons (bottom). Short arrows indicate non-specified axonal or neuronal injuries. Long solid or dashed arrows indicate normal or compromised axonal transport (respectively).
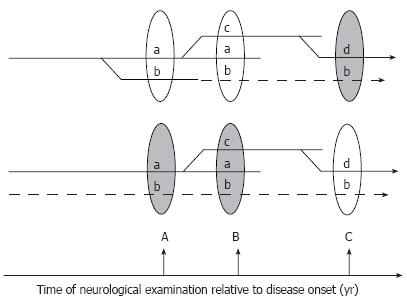
Figure 5 Hypothetical branching (top) and multi-trigger (bottom) pathogenesis of DPN.
In the first scenario (top) all manifestations of the disease result from the unique branching pathogenic process, and symptom “b” discovered at the time of neurological exam B is not corrected by treatment because it has already progressed to an irreversible stage (dashed lines). Earlier diagnosis and institution of treatment (at time A) may critically change the outcome of therapy in this scenario. Alternatively (lower scenario), several independent factors may trigger and maintain the progression of DPN. In this case the therapy may fail to treat symptoms not because they are irreversible, but because the correct cause of the pathology is not identified and is not treated (dashed lines).
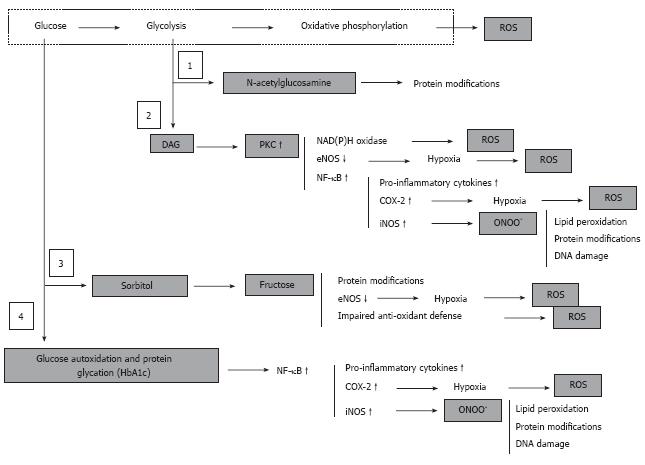
Figure 6 Hyperglycemia, derangement of cell metabolism and oxidative/nitrosative stress.
Hyperglycemia associates with accumulation of fructose-6-phosphate and hexosamine pathway (1) to N-acetylglucosamine, accumulation of dihydroxyacetone phosphate and associated activation of PKC (2), activation of polyol sugar pathway (3), and glucose autoxidation and non-enzymatic protein glycation (4). These metabolic events are either regular physiologically important components cell metabolism (1, 2 and 3) or are normally under strict control of intrinsic intracellular defense mechanisms (4). However, under conditions of chronic hyperglycemia activation of these pathways leads to a global derangement of the cell and tissue homeostasis, which culminates in an uncontrolled cascade of abnormal protein modifications, oxidative/nitrosative stress and pro-inflammatory conditions.
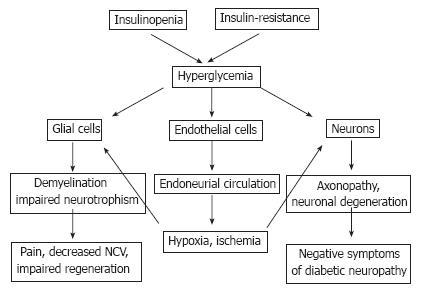
Figure 7 Pathogenesis of DPN with hyperglycemia as a major trigger of PNS injury.
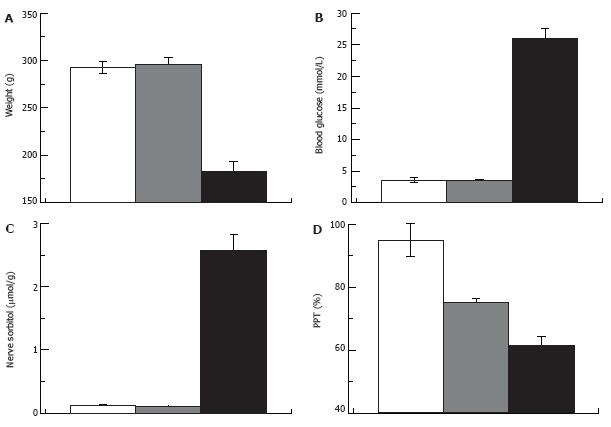
Figure 8 Pain pressure thresholds are not correlated with hyperglycemia.
Weight (A), blood glucose (B), nerve sorbitol (C) and pain pressure thresholds (D) in control, STZ-normoglycemic and hyperglycemic rats (white, grey and black columns respectively; 2 wk after injection of 65 mg/kg STZ).
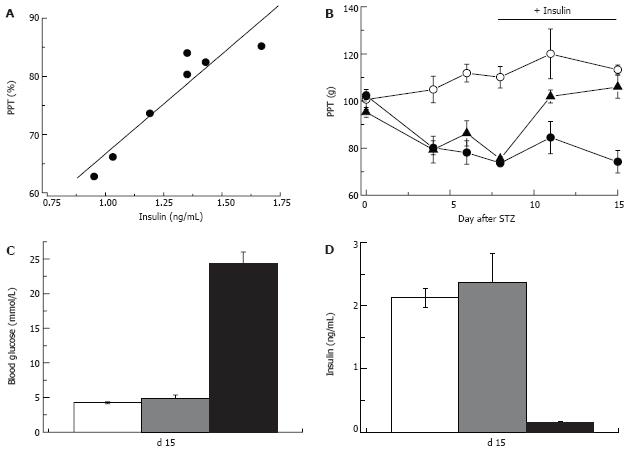
Figure 9 Insulin-dependence of pain pressure threshold in STZ-normoglycemic rats.
Two weeks after injection of STZ pain pressure threshold of STZ-NG rats is decreased in proportion to the plasma insulin level (R = 0.97; A). Insulin replacement initiated one week after STZ injection (horizontal bar in panel B) does not change PPT of control or hyperglycemic rats, but corrects it in STZ-normoglycemic animals (empty and filled circles, and filled triangles, respectively). Insulin replacement, does not affect blood glucose (C), but normalizes plasma insulin level (D) in STZ-NG rats. In C and D white, grey and black columns represent control, STZ-NG and STZ-HG rats, respectively.
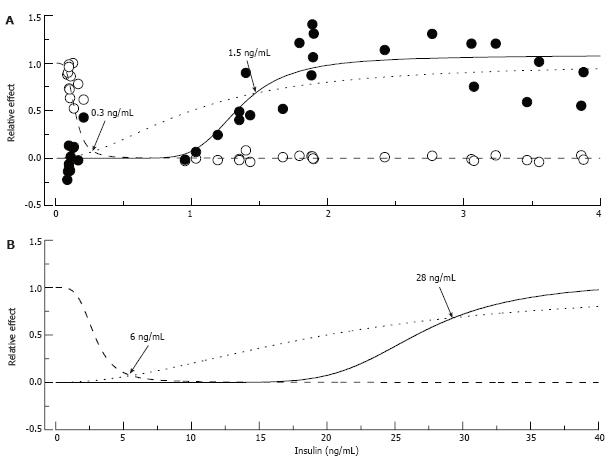
Figure 10 Putative “insulin-glucose metabolism” and “insulin-nerve function” relationships in normal and insulin-resistant rats.
In A: Normal rats: Empty circles represent fasting glucose and filled circles pain pressure thresholds measured in control, STZ-NG and STZ-HG rats, pooled together and normalized to show relative changes of these parameter between control and diabetic animals. The data were fitted by Hill curves calculated based on the assumption that insulin binds to the receptor with an affinity of 1 ng/mL (dotted curve) and 10% and 65% occupancy of these receptors is required for maximum metabolic (intersection with dashed curve) and nerve (intersection with solid curve) effect of the hormone, respectively. In B: Insulin-Resistant Rats: Insulin resistant state was simulated by increasing K0.5 of insulin binding to the receptor to 20 ng/mL. Points of intersections of the dotted curve with the dashed and solid curves recalculated with the new K0.5 parameter show (arrows and labels) that maintaining glucose metabolism now requires about 6 ng/mL of plasma insulin, and nerve function requires at least 28 ng/mL of the hormone.
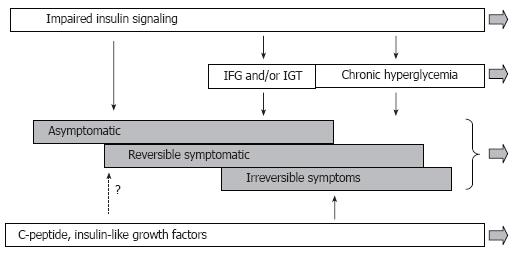
Figure 11 Modified scheme of pathogenesis of diffuse diabetic neuropathy with inclusion of insulin signaling.
According to this view, the disease process starts before impairment of glucose metabolism becomes apparent. Derangement of insulin signaling in PNS triggers and maintains the neuropathy at this stage. Postprandial and chronic hyperglycemia is not the least important factor of DPN, but they become involved at relatively advanced stages of DPN. C-peptide and IGFs insufficiencies represent another important set of pathogenic mechanisms; however, the role of these mechanisms in early DPN remains undetermined.
- Citation: Dobretsov M, Romanovsky D, Stimers JR. Early diabetic neuropathy: Triggers and mechanisms. World J Gastroenterol 2007; 13(2): 175-191
- URL: https://www.wjgnet.com/1007-9327/full/v13/i2/175.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i2.175