Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 21, 2006; 12(7): 1092-1097
Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1092
Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1092
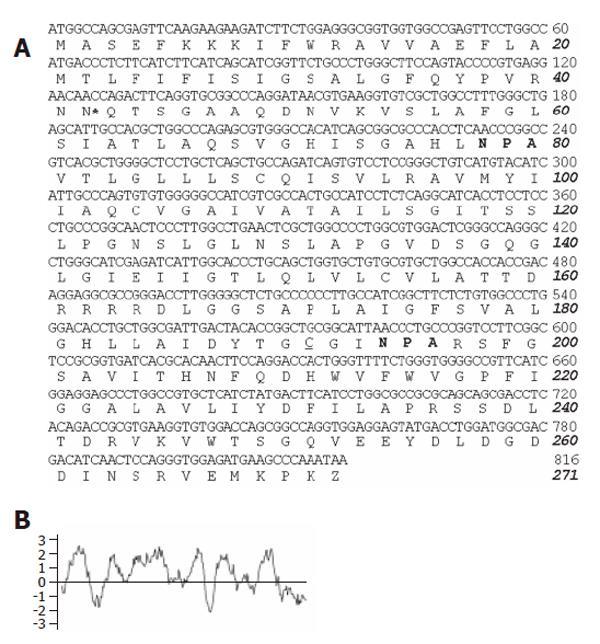
Figure 1 cDNA and deduced amino acid sequences of pig AQP1 water channel.
A: Two NPA sequences conserved in water channel proteins of aquaporin family (in bold). *indicates the consensus sequence for N-linked glycosylation and the mercurial-sensitive site cysteine 201 near the second NPA motif is underlined; B: Kytte-Doolittle hydropathy profile of the deduced pAQP1 amino acid sequence.
Figure 2 Amino acid sequence alignment of pAQP1 with its dog, human and mouse orthologs.
Conserved amino acids are shielded, NPA motifs are boxed and cycteines near the second NPA motifs are underlined. The consensus N-linked glycosylation sites are shown in bold.
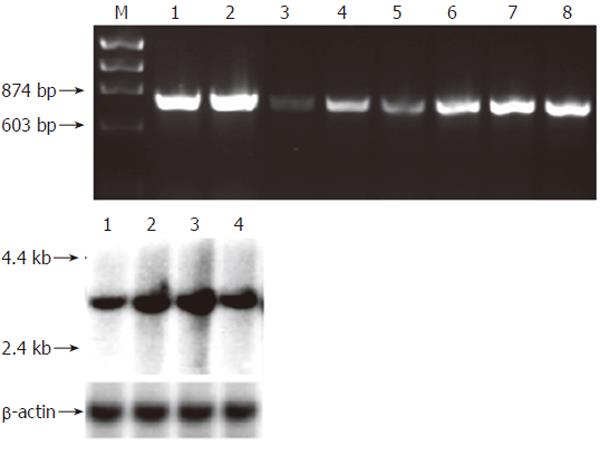
Figure 3 mRNA expression of pAQP1 water channel in pig gastrointestinal organs.
A: Expression of pAQP1 transcript by RT-PCR analysis. Lanes 1, Salivary gland; 2, Liver; 3, Pancreas; 4, Esophagus; 5, Stomach; 6, Jejunum; 7, Ileum; 8, Colon; B: Northern blotting of total RNA (20 µg) from salivary gland (lane 1), liver (lane 2), ileum (lane 3) and colon (lane 4) probed with the pAQP1 coding sequence (upper) and the same membrane probed with a 600 bp pig β-actin cDNA sequence (lower).
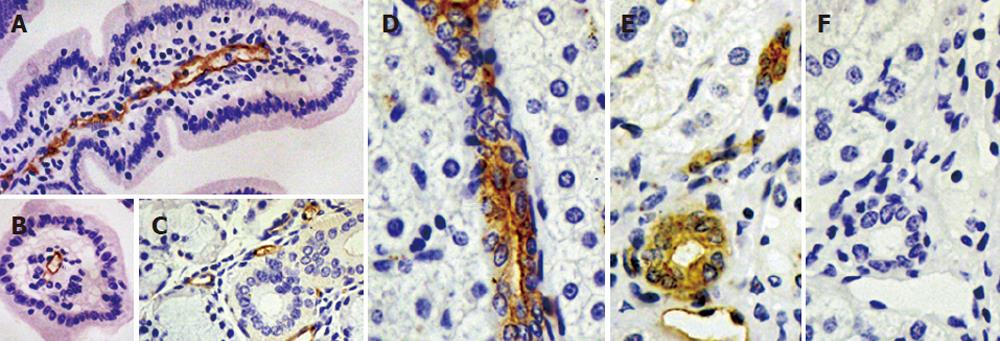
Figure 4 Immunolocalization of pAQP1 in pig gastrointestinal tract and exocrine glands.
Specific pAQP1 labeling of central lacteals was seen by immunoperoxidase staining of longitudal (A) and traverse (B) sections of the small intestinal villi using an affinity-purified AQP1 antibody. Arrows indicate the endothelium of central lacteals; A salivary gland section showing specific labeling of microvessel endothelium indicated by arrows (C); Specific pAQP1 labeling was seen in the epithelium of intrahepatic bile ducts in longitudal (D) and traverse (E) sections. Arrows indicate heavy pAQP1 staining in the apical domain of bile duct epithelial cells. Arowhead indicates pAQP1 labeling in the endothelium of periductal microvessels; A consecutive section of E showing immunostaining with AQP1 antibody preabsorbed with the immunizing peptide (F).
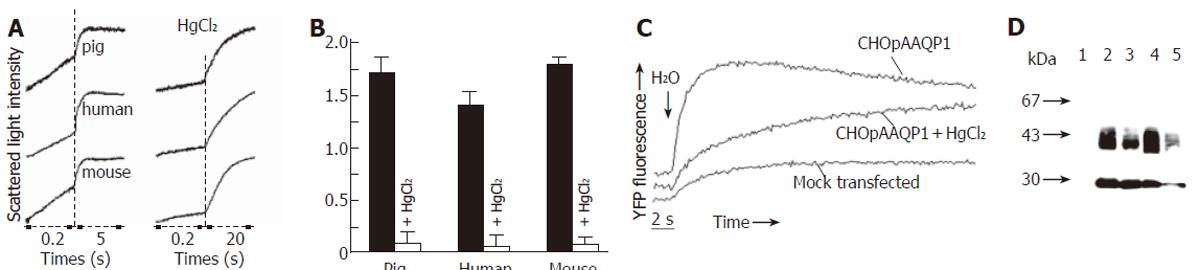
Figure 5 Functional properties of pAQP1 in erythrocytes and stably transfected CHO cells.
A: Osmotic water permeability of pig, human and mouse erythrocytes measured by stopped-flow light scattering from the time course of erythrocyte volume in response to a 100 mmol/L inwardly directed sucrose gradient in the absence (left panel) and presence (right panel) of 0.3 mmol/L HgCl2; B: Summary of osmotic water permeability coefficient (Pf) for erythrocytes from pig, human and mouse measured in A (mean ± SE, n = 4); C: Osmotic water permeability of CHO cells stably transfected with pAQP1 cDNA measured by YFP-based fluorescence assay. Water permeability of CHO cells was expressed as half time (t1/2) needed from water injection to the point when the cytoplasmic fluorescence reached the maximum. t1/2 of pAQP1 transfected CHO cells in the absence and presence of 0.1 mmol/L HgCl2 was 1.2 s and 4.6 s separately. t1/2 > 20 s in mock-transfected CHO cells; D: Immunoblot of erythrocytes and transfected CHO cells. Lanes 1 - 4: erythrocytes from AQP1-/- mice, AQP1 +/+ mice, pigs and human beings. Lane 5: CHO cells stably transfected with pAQP1 cDNA.
- Citation: Jin SY, Liu YL, Xu LN, Jiang Y, Wang Y, Yang BX, Yang H, Ma TH. Cloning and characterization of porcine aquaporin 1 water channel expressed extensively in gastrointestinal system. World J Gastroenterol 2006; 12(7): 1092-1097
- URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1092.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1092