Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 7, 2006; 12(5): 784-790
Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.784
Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.784
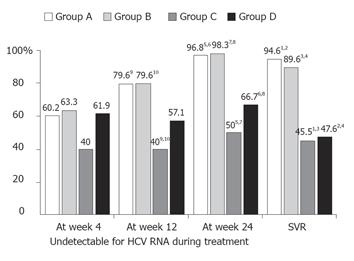
Figure 1 The sustained virological response (SVR) rate and undetectable hepatitis C virus (HCV) RNA rates during the treatment of 173 patients, classified by continuation and discontinuation of interferon and ribavirin combination treatment.
Group A patients (n = 93) who well tolerated the 24-week treatment with IFN and ribavirin in combination without any reduction in the dose of either drug; Group B patients (n = 48) received the 24-week combination treatment, but needed a dose reduction of IFN or ribavirin, or both; Group C patients (n = 11) discontinued the ribavirin treatment, but continued the full 24 weeks of IFN treatment; Group D patients (n = 21) did not complete the 24 weeks of treatment because of adverse effects (n = 17) or dropped out (n = 4). 1P = 0.0001; 2P < 0.0001; 3P = 0.0031; 4P = 0.0003; 5P = 0.0001; 6P = 0.0002; 7P = 0.0003; 8P = 0.0002; 9P = 0.124; 10P = 0.0182.
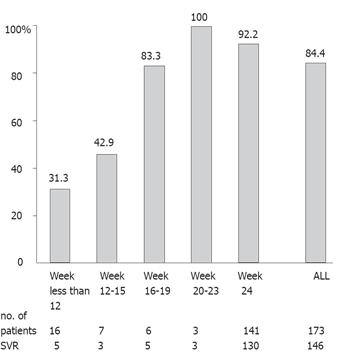
Figure 2 Relationship between the sustained virological response rates and the length of ribavirin treatment period of the 173 studied patients.
- Citation: Furusyo N, Katoh M, Tanabe Y, Kajiwara E, Maruyama T, Shimono J, Sakai H, Nakamuta M, Nomura H, Masumoto A, Shimoda S, Takahashi K, Azuma K, Hayashi J, Group KULDS. Interferon alpha plus ribavirin combination treatment of Japanese chronic hepatitis C patients with HCV genotype 2: A project of the Kyushu University Liver Disease Study Group. World J Gastroenterol 2006; 12(5): 784-790
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.784